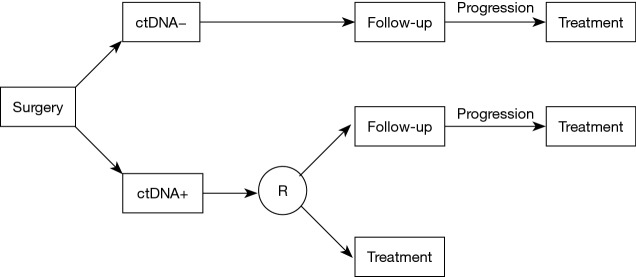
Figure 1.
Design of a clinical trial investigating the benefit of ctDNA testing in the adjuvant setting. Following surgery, patients will be tested on plasma. If the test is negative, the patients will be followed until progression, and then treated with adjuvant therapy. If the initial plasma test is positive, patients will be randomized. In the control arm, the patients will be followed until progression, and then treated. In the experimental arm, the patients will be treated with adjuvant therapy directly. ctDNA, circulating tumor DNA.