Figure 2.
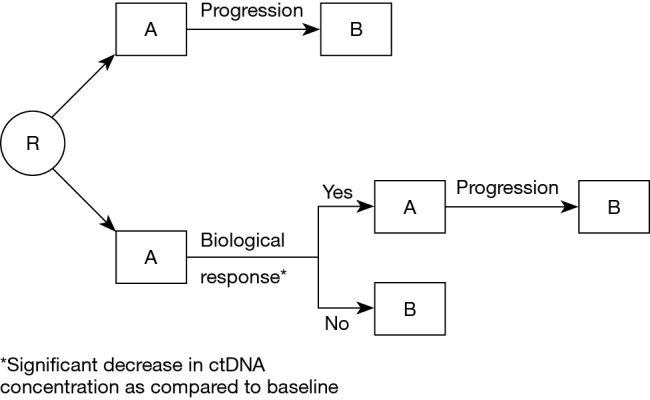
Design of a clinical trial investigating the potential of ctDNA monitoring to guide therapy in the metastatic setting. In the reference arm, patients will be treated with A, then with B following radiological or clinical progression. In the experimental arm, early ctDNA analysis (after for instance 2 or 3 weeks of treatment), will allow to identify a change in ctDNA concentration as compared to the pre-treatment assay performed. In case of significant decrease, suggesting that the patient is responding, treatment A will be continued until radiological or clinical progression. If there is no decrease in ctDNA concentration, suggesting that the patient is not responding to treatment A, the treatment will be changed to B. ctDNA, circulating tumor DNA.
