Abstract
Xanthan gum is an important commercial polysaccharide produced by Xanthomonas species. In this study, xanthan production was investigated using a local isolate of Xanthomonas campestris MO-03 in medium containing various concentrations of chicken feather peptone (CFP) as an enhancer substrate. CFP was produced with a chemical process and its chemical composition was determined. The addition of CFP (1–8 g/l) increased the conversion of sugar to xanthan gum in comparison with the control medium, which did not contain additional supplements. The highest xanthan production (24.45 g/l) was found at the 6 g/l CFP containing control medium in 54 h. This value was 1.73 fold higher than that of control medium (14.12 g/l). Moreover, addition of CFP improved the composition of xanthan gum; the pyruvate content of xanthan was 3.86% (w/w), higher than that of the control (2.2%, w/w). The xanthan gum yield was also influenced by the type of organic nitrogen sources. As a conclusion, CFP was found to be a suitable substrate for xanthan gum production.
Keywords: Chicken feather peptone, Polysaccharide, Pyruvate, Xanthomonas campestris, Xanthan gum
1. Introduction
Xanthan is a water-soluble hetero-exopolysaccharide produced by the Gram-negative plant pathogenic bacterium Xanthomonas campestris. Xanthan gum shows a wide range of applications such as suspending, stabilizing, thickening and emulsifying agent in the food, cosmetics, pharmaceutical, paper, paint, textile and oil industries [1], [2], [3]. Xanthan production is constantly increasing because of its numerous applications. It is estimated that the annual global production of xanthan gum is over 80.000 tonnes worth $400 million [4].
The production of xanthan gum has been shown to be influenced by many factors such as species type and environmental factors including dissolved oxygen level, media composition, temperature, pH and incubation time [1], [5], [6]. A cost reduction in xanthan gum production can be achieved by using inexpensive sources such as molasses [4], cheese whey [7], starch [8], kitchen waste [9], glycerol [10], coconut shell, passion fruit peel, corn straw and cobs [11] and jackfruit seed powder [12]. These materials have been used as a carbon source in submerged or solid state fermentations. Also, the type and concentration of nitrogen source affects xanthan fermentation [13]. Especially, organic nitrogen sources have been found to be better than inorganic nitrogen sources for xanthan production. Peptone [14], yeast extract, corn steep liquor [15] and ram horn peptone [13] have been used as organic nitrogen source in xanthan gum fermentations. Therefore, there is a need for cheap and available organic nitrogen sources.
Feathers are produced several million tons as a waste in poultry-processing plants. A large amount of feather waste is not recycled as required in nature and so it cause environmental pollution. About 10% of total chicken weight is feathers. Feathers are consist of approximately 90% protein composed of keratin thus can be a potential source of proteins and amino acids [16], [17]. Considering these properties, feathers are cheap and available bioorganic waste to peptone production. Recently, the chicken feather peptone or hydrolysates have been used as a complex nitrogen source for the production of lactic acid [18], biosurfactant [19], polyhydroxyalkanoate [20] and citric acid [21].
In this study, it is the first time that CFP has been tested as an enhancer for X. campestris MO-03 to the production of xanthan. This study was aimed at the development of economical methods for higher yields of xanthan by suggesting the use of low cost raw material.
2. Material and methods
2.1. Hydrolysis of chicken feather
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jgeb.2018.07.005.
The chemicals used in this study were analytical grade and purchased from Sigma–Aldrich (St Louis, MO, USA) and Difco (Detroit, MI, USA). Chicken feathers were obtained from the Demircioglu Poultry Farm, Zonguldak, Turkey. Feathers were washed with deionized water and dried in oven at 60 °C. Dried feathers were cut into smaller pieces and then they were powdered with a blender. This material was hydrolysated by modifying the method of Kurbanoglu and Kurbanoglu [22], and the production process of CFP is shown in Fig. S1.
Fig. S1.
A production scheme for CFP.
2.2. Isolation and identification of xanthan gum producing bacterium
Xanthomonas species were isolated from infected plants using Yeast Malt extract (YM) agar (KH2PO4 5 g/l, yeast extract 4 g/l, MgSO4 0.5 g/l, malt extract 2 g/l, glucose 10 g/l and agar 15 g/l, pH 7). The yellow-mucoid bacterial colonies were selected and maintained on Nutrient Agar (NA) slants. Bacterial isolates were identified by various tests, such as the Gram staining, catalase and oxidase tests, and morphology. Analysis of 16S rDNA was performed according to Gur et al. [23] for the best xanthan producing isolate.
2.3. Media
One loop of cells grown on YM agar plates for three days was used to inoculate a 250 ml flask containing 50 ml of YM broth. The shake flasks were incubated at 28 °C and 200 rpm for 24 h. Five milliliters of the inoculum culture were added to 100 ml production medium in a 500 ml erlen flask. The control medium composition was as follows (g/l): glucose 40, citric acid 2.1, NH4NO3 1.14, KH2PO4 2.87, MgCl2 0.5, Na2SO4 0.09, H3BO3 0.0006, FeCl3 0.020 and 0.03 ml/L concentrated HCl [13], [24]. In order to determine the effects of CFP on the xanthan gum production, 0 (control medium, CM), 1–8 g/l CFP were added to the production medium, respectively. The pH was adjusted to 7.0 before autoclaving at 121 °C for 15 min. The culture temperature and agitation rate were maintained at 30 °C and 200 rpm, respectively. Later, CFP was compared with three commercial organic nitrogen sources (yeast extract, bacto peptone, and tryptone) at the concentration, which was determined as optimal for CFP.
2.4. Analytical methods
Total sugar, dry matter and ash contents of CFP were estimated by AOAC methods [25]. Nitrogen content was determined using a micro-kjeldahl apparatus (Labconco corporation, USA). Amino acids were analyzed using reverse-phase high performance liquid chromatography (C18 column, 3.9 mm × 15 cm). Crude fat content was measured with a Soxhlet apparatus using diethyl ether. At regular intervals (18 h) of fermentation, the microbial growth, residual sugar and xanthan gum were determined. For biomass estimation, cultures were harvested by centrifugation, washed twice with sterile distilled water and dried at 80 °C until constant weight was achieved. The dinitrosallicylic acid (DNS) method of Miller [26] was employed for residual sugar. Cell-free supernatant was mixed with ethanol (1:2 v/v) to precipitate the xanthan gum. The precipitated xanthan gum was separated and dried in oven at 90 °C until constant weight [13]. Pyruvate content of xanthan gum was determined by reaction with 2,4 dinitrophenylhydrazine according to Sloneker and Orentas [27].
2.5. Statistical analysis
Experiments were replicated three times in a randomized block design. The statistical analyses of the data were performed one-way analysis of variance (ANOVA). The level of significance was P < 0.05. All statistical analyses were performed using SPSS 15.0 software programme.
3. Results and discussion
3.1. Production and chemical analysis of CFP
As shown in Fig. S1, 100 g chicken feather was hydrolyzed with HCl and H2SO4. NaOH, KOH, Mg(OH)2 and Ca(OH)2 were used for the neutralization of hydrolysates. The main chemical composition of CFP is shown in Table S1. It was detected that CFP had high protein (56 g 100 g−1), ash (41.5 g 100 g−1), nitrogen (9 g 100 g−1) and low fat (0.2 g 100 g−1) contents. CFP contained all of amino acids, except methionine and tryptophan (destroyed by acid hydrolysis), at varying concentrations and was especially rich in alanine (3.758 g 100 g−1), leucine (5.019 g 100 g−1), glutamate (6.107 g 100 g−1), glycine (5.453 g 100 g−1), serine (4.250 g 100 g−1) and proline (8.106 g 100 g−1). As seen in Table S1, CFP was also rich especially in Ca, K, Mg, S and Na because of the hydrolysis processes. Similar results have been obtained in previous studies [18], [19], [21], [22].
Supplementary Table S1.
Composition of CFP produced from chicken feather.
3.2. Isolation and identification of Xanthomonas isolates
The Xanthomonas sp. strains, plant pathogens, were isolated from the infected leaves of different plants. Primary identification of the Xanthomonas sp. strains (mucoid colonies) were conducted according to pigment production. Several species of bacteria contained identical pigments [28] and X. campestris produces yellow pigment called xanthomonadin [29]. Xanthomonas sp. strains were formed yellow pigmented slimy or mucoid colonies on YMA and NA plates. Morphological and classical tests showed that they were gram negative, aerobic, rod shaped, oxidase negative, catalase positive, mobile organisms. The isolated four cultures of Xanthomonas sp. were screened to obtain the best xanthan gum producer strain. Among the all strains, MO-03 produced maximum xanthan gum and biomass 14.45 g/l and 2.74 g/l, respectively. The xanthan gum from strain MO-02 had lower pyruvic acid content than other isolates (Table 1). The most xanthan producing strain, Xanthomonas campestris strain MO-03, was identificated according to 16S rDNA sequencing analysis. Finally, 1488 bp 16S rDNA sequence of the strain was BLAST searched and the sequence was deposited in GenBank with the accession number of KF939142.
Table 1.
Growth, xanthan production and pyruvate contents of xanthan by the isolates in control medium for 72 h.
| Isolates | Biomass (g/L) | Xauthan gum (g/L) | Pyruvate (%, w/w) |
|---|---|---|---|
| Xanthomonas sp. MO-01 | 2.15 | 11.23 | 1.9 |
| Xanthomonas sp. MO-02 | 2.32 | 7.65 | 1.5 |
| Xanthomonas sp. MO-03 | 2.70 | 14.56 | 2.2 |
| Xanthomonas sp. MO-04 | 2.40 | 9.32 | 1.8 |
3.3. Effect of CFP on xanthan gum production
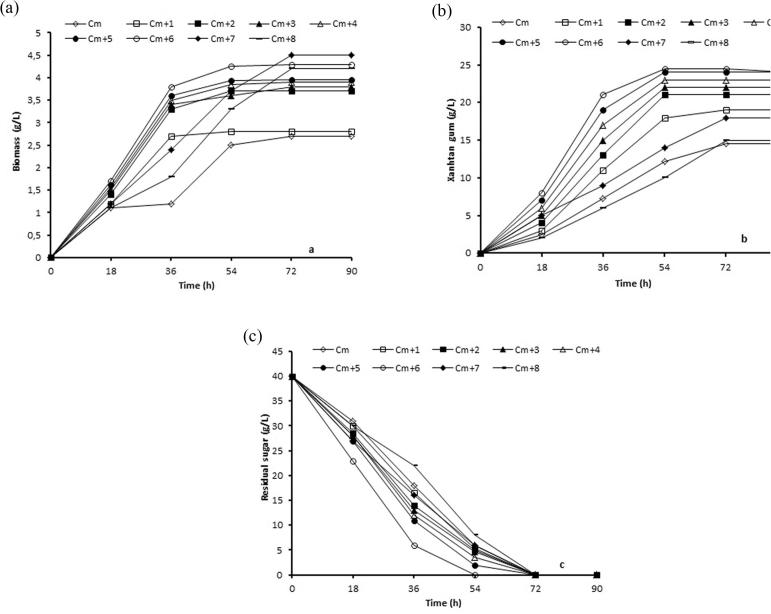
Fig. 1 shows the effect of the adding CFP (1–8 g/l) to the control medium (CM) on xanthan fermentation. The addition of CFP (1–8 g/l) to the CM increased microbial biomass, sugar consumption and xanthan gum formation. As seen in Fig. 1a, addition of CFP at 7 g/l gave the highest biomass yield of 4.5 g/l at 90 h. However, X. campestris MO-03 produced a maximum of 24.45 g/l of xanthan gum at 54 h in the presence of 6 g/l CFP while the maximum xanthan concentration in the CM was 14.56 g/l at 72 h (Fig. 1b) with the complete depletion of sugar contents in these media (Fig. 1c). Increasing the concentration of CFP from 0 to 6 g/l in CM increased the xanthan gum yield from 12.2 g/l to 24.45 g/l for 54 h. This value is 2 fold higher as compared to CM. Xanthan gum yields based on sugar consumption were calculated to be 61.12% and 30.5% for the CM + 6 g/l CFP and CM, respectively. Obviously, the addition of CFP greatly stimulated the conversion of sugar to xanthan gum and also resulted in reduced fermentation time. The maximum xanthan gum production was obtained in the exponential growth phase (Fig. 1). Similar results were also confirmed by Faria et al. [4] and Savvides et al. [30]. In this study, it was determined that CFP concentration greater than 6 g/l CFP was inhibitory to xanthan production. This inhibitory effect may be due to high salt concentration, some toxic materials in CFP and, change of the C/N rate of culture medium [31], [32], [33].
Fig. 1.
Comparison of the effect of CFP in various concentrations for different incubation times: (a) biomass concentration, (b) xanthan concentration and (c) sugar utilization.
It was reported that the presence of glutamate, aspartate, proline, hydroxyproline, threonine and alanine stimulated xanthan gum production [33]. Also, Murad et al. [14] found that several amino acids (cysteine, histidine, glycine and serine) were suitable for xanthan gum production. To produce xanthan gum, X. campestris needs several nutrients, including micronutrients (e.g. K, Fe, P, Mg, S and Ca salts) and macronutrients (C and N) [1], [33]. The CFP was considered as an enhancer of xanthan gum production because of including these amino acids and minerals or salts.
The quality of xanthan depends on its pyruvate content [15]. Fig. 2 demonstrates that the pyruvate content of xanthan gum depends on concentration of the CFP. The pyruvate contents of xanthan gum measured at their maximum production times. There was a significant difference in pyruvate content of xanthan gum between the media (P < 0.05). It was found that the content of pyruvate increased in proportion to CFP concentration. Similarly, researchers reported that presence of ram horn hydrolysate increases the pyruvilation degree [13]. But an increase in the initial inorganic nitrogen concentration reduces the amount of pyruvate [1], [35], [35]. Moreover, the extent of pyruvate of xanthan gum depends on the fermentation parameters such as media composition, oxygen, temperature, pH, incubation time and H2O2 [1], [6], [34].
Fig. 2.
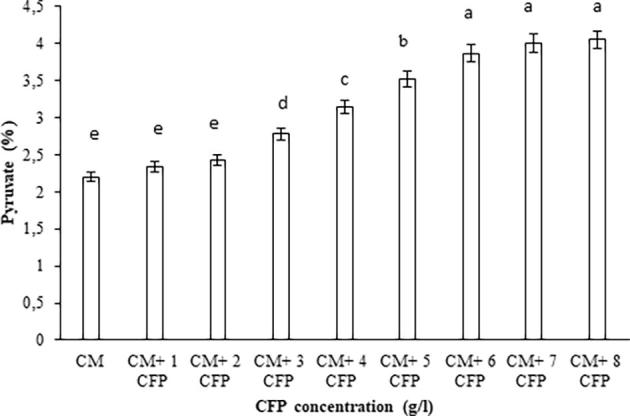
Effect of the CFP on the pyruvate content of xanthan gum. Values with the same letter are not significant (P < 0.05).
3.4. Effect of organic nitrogen sources on production of xanthan
The type of nitrogen sources are very critical on growth and xanthan production by X. campestris [14], [36]. Fig. 3 shows the marked stimulatory effect of organic nitrogen sources on xanthan synthesis. The results demonstrated that the maximum biomass yield was obtained with TP (4.88 g/l). Xanthan production (24.45 g/l) was the best when CFP was used as supplement nitrogen source, among all the organic nitrogen sources (tryptone TP, bacto peptone BP, and yeast extract YE) tested (Fig. 3). The least xanthan was obtained in the CM containing BP (17.9 g/l). This positive effect of CFP may be a result of high amino acid and mineral contents. Many researchers reported that the addition of organic nitrogen sources (yeast extract, ram horn hydrolyzate, peptone) to production medium promoted cell growth, and xanthan production [8], [13], [14], [37].
Fig. 3.
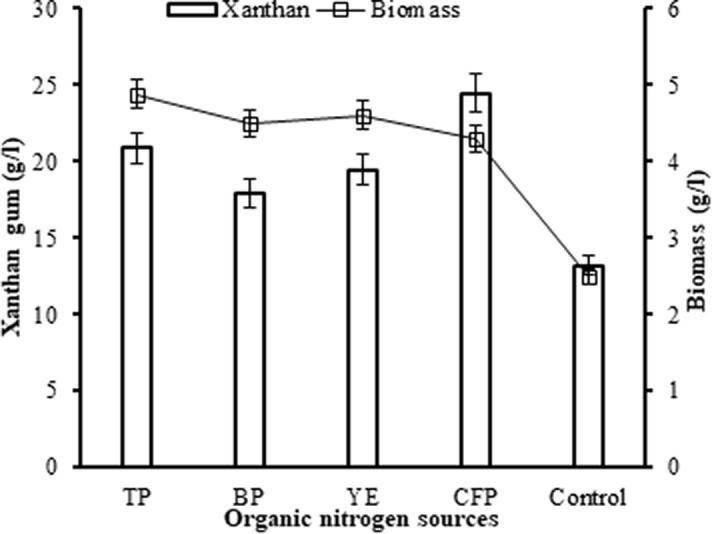
Effect of the organic nitrogen sources on the xanthan gum and microbial biomass. Culture conditions: Initial pH 7.0, 200 rpm, 30 °C, 54 h. TP: Tryptone, BP: Bacto peptone, YE: Yeast extract, CFP: Chicken feather peptone.
As seen in Fig. 4, the addition of organic nitrogen sources significantly improved (P > 0.05) the quality of xanthan gum; the pyruvate content of xanthan was 3.2–4.05% (w/w), higher than that of the control medium (2.2%, w/w). The results showed that the maximum pyruvate yields were obtained with YE and CFP. Organic nitrogen sources were found to be good nitrogen sources for the production of xanthan gum with higher pyruvate contents [13], [38]. As mentioned above, the stimulatory effect of these organic nitrogen sources may be due to the availability of soluble amino acids and minerals in the fermentation broth.
Fig. 4.
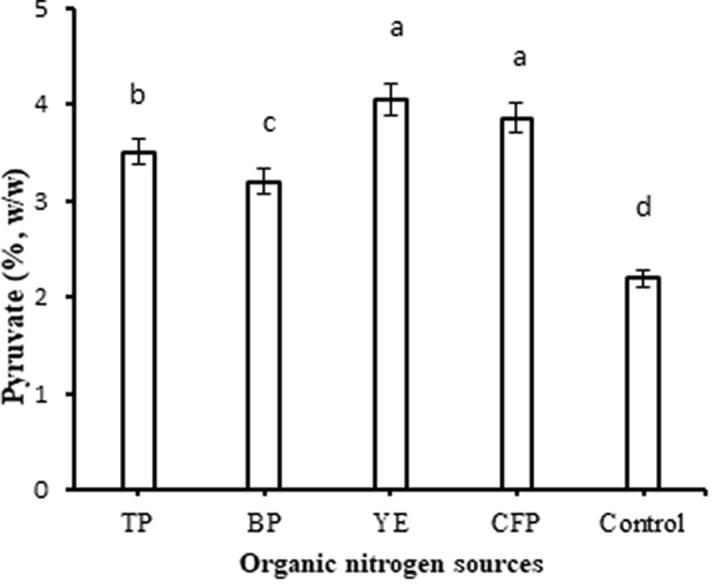
Effect of the organic nitrogen sources on the pyruvate content of xanthan gum. Culture conditions: Initial pH 7.0, 200 rpm, 30 °C, 54 h. TP: Tryptone, BP: Bacto peptone, YE: Yeast extract, CFP: Chicken feather peptone.
In conclusion, organic nitrogen sources are known to be better nitrogen sources for xanthan production compared to inorganic nitrogen sources. But, commercial organic nitrogen sources are expensive. Therefore, waste chicken feathers were converted to CFP through chemical processes. Chicken feathers are a renewable, inexpensive and easily available waste in Turkey and world. Utilization of CFP to produce xanthan gum appears to be economic. The xanthan synthesis was greater in the presence of CFP as compared with commercial organic nitrogen sources. The addition of the CFP greatly increased the bioconversion of sugar into xanthan by X. campestris MO-03. Consequently, CFP is an enhancer for xanthan gum production with high pyruvate content.
Acknowledgments
Acknowledgments
This research was supported by a grant from the research funds appropriated to Ataturk University, Erzurum, Turkey.
Conflicts of interest
No conflict of interest declared.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Garcia-Ochoa F., Santos V.E., Casas J.A., Gomez E. Xanthan gum: Production, recovery, and properties. Biotechnol Adv. 2000;18:549–579. doi: 10.1016/s0734-9750(00)00050-1. [DOI] [PubMed] [Google Scholar]
- 2.Petri D.F. Xanthan gum: a versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015;132:42035. [Google Scholar]
- 3.Kumar A., Rao K.M., Han S.S. Application of xanthan gum as polysaccharide in tissue engineering: a review. Carbohydr Polym. 2017;180:128–144. doi: 10.1016/j.carbpol.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Faria S., Vieira P.A., Resende M.M., França F.P., Cardoso V.L. A comparison between shaker and bioreactor performance based on the kinetic parameters of xanthan gum production. Appl Biochem Biotechnol. 2009;156:45–58. doi: 10.1007/s12010-008-8485-8. [DOI] [PubMed] [Google Scholar]
- 5.Kalogiannis S., Iakovidou G., Liakopoulou-Kyriakides M., Kyriakidis D.A., Skaracis G.N. Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process Biochem. 2003;39:249–256. [Google Scholar]
- 6.Cheng R., Lin L., Zhang Y. Hydrogen peroxide (H2O2) supply significantly improves xanthan gum production mediated by Xanthomonas campestris in vitro. J Ind Microbiol Biotechnol. 2012;39:799–803. doi: 10.1007/s10295-011-1071-z. [DOI] [PubMed] [Google Scholar]
- 7.Niknezhad S.V., Asadollahi M.A., Zamani A., Biria D., Doostmohammadi M. Optimization of xanthan gum production using cheese whey and response surface methodology. Food Sci Biotechnol. 2015;24:453–460. [Google Scholar]
- 8.Bhatia S.K., Kumar N., Bhatia R.K. Stepwise bioprocess for exopolysaccharide production using potato starch as carbon source. 3 Biotech. 2015;5:735–739. doi: 10.1007/s13205-014-0273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P., Li T., Zeng Y., Li X., Jiang X., Wang Y., Xie T., Zhang Y. Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr Polym. 2016;151:684–691. doi: 10.1016/j.carbpol.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z., Wu J., Gao M.J., Zhu L., Zhan X.B. High production of xanthan gum by a glycerol-tolerant strain Xanthomonas campestris WXLB-006. Prep Biochem Biotechnol. 2017;47:468–472. doi: 10.1080/10826068.2017.1292288. [DOI] [PubMed] [Google Scholar]
- 11.dos Santosa F.P., Jra A.M.O., Nunesa T.P., de Farias Silvab C.E., de Souza Abud A.K. Bioconversion of agro-industrial wastes into xanthan gum. Chem Eng Trans. 2016;49:145–150. [Google Scholar]
- 12.Katherine R.F., Muthukumaran C., Sharmila G., Kumar N.M., Tamilarasan K., Jaiganesh R. Xanthan gum production using jackfruit-seed-powder-based medium: optimization and characterization. Biotech. 2017;7:248. doi: 10.1007/s13205-017-0876-5. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurbanoglu E.B., Kurbanoglu N.I. Ram horn hydrolysate as enhancer of xanthan production in batch culture of Xanthomonas campestris EBK-4 isolate. Process Biochem. 2007;42:1146–1149. [Google Scholar]
- 14.Murad H.A., Mohamed S.H., Abu-El-Khair A.G. Impact of amino acids, nitrogen source and buffering system on xanthan yield produced on hydrolyzed whey lactose. Biotechnology. 2017;16:69–76. [Google Scholar]
- 15.De Vuyst L., Vermeire A. Use of industrial medium components for xanthan production byXanthomonas campestris NRRL-B-1459. Appl Microbiol Biotechnol. 1994;42:187–191. [Google Scholar]
- 16.Kamarudin N.B., Sharma S., Gupta A., Kee C.G., Chik S.M.S.B.T., Gupta R. Statistical investigation of extraction parameters of keratin from chicken feather using design-expert. 3 Biotechnology. 2017;7:127. doi: 10.1007/s13205-017-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maciel J.L., Werlang P.O., Daroit D.J., Brandelli A. Characterization of protein-rich hydrolysates produced through microbial conversion of waste feathers. Waste Biomass Valor. 2017;8:1177–1186. [Google Scholar]
- 18.Taskin M., Esim N., Ortucu S. Efficient production of l-lactic acid from chicken feather protein hydrolysate and sugar beet molasses by the newly isolated Rhizopus oryzae TS-61. Food Bioprod Process. 2012;90:773–779. [Google Scholar]
- 19.Ozdal M., Gurkok S., Ozdal O.G. Optimization of rhamnolipid production by Pseudomonas aeruginosa OG1 using waste frying oil and chicken feather peptone. Biotechnology. 2017;7:117. doi: 10.1007/s13205-017-0774-x. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benesova P., Kucera D., Marova I., Obruca S. Chicken feather hydrolysate as inexpensive complex nitrogen source for PHA production by Cupriavidus necator on waste frying oils. Lett Appl Microbiol. 2017;65:182–188. doi: 10.1111/lam.12762. [DOI] [PubMed] [Google Scholar]
- 21.Ozdal M., Kurbanoglu E.B. Citric acid production by Aspergillus niger from agro-industrial by-products: molasses and chicken feather peptone. Waste Biomass Valor. 2018 [Google Scholar]
- 22.Kurbanoglu E.B., Kurbanoglu N.I. Ram horn peptone as a source of citric acid production by Aspergillus niger, with a process. J Ind Microbiol Biotechnol. 2004;31:289–294. doi: 10.1007/s10295-004-0147-4. [DOI] [PubMed] [Google Scholar]
- 23.Gur O., Ozdal M., Algur O.F. Biodegradation of the synthetic pyrethroid insecticide α-cypermethrin by Stenotrophomonas maltophilia OG2. Turk J Biol. 2014;38:684–689. [Google Scholar]
- 24.Jana A., Ghosh P. Stimulation of xanthan production by Xanthomonas campestris using citric acid. World J Microbiol Biotechnol. 1997;13:261–264. [Google Scholar]
- 25.AOAC . 13th ed. Association of Official Agricultural Chemists; Washington, D.C.: 1980. Official methods of analysis. [Google Scholar]
- 26.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 27.Sloneker J.H., Orentas D.G. Pyruvic acid, a unique component of an exocellular bacterial polysaccharide. Nature. 1962;104:478–479. doi: 10.1038/194478a0. [DOI] [PubMed] [Google Scholar]
- 28.Okay S., Ozdal M., Kurbanoǧlu E.B. Characterization, antifungal activity, and cell immobilization of a chitinase from Serratia marcescens MO-1. Turk J Biol. 2013;37:639–644. [Google Scholar]
- 29.Tuli H.S., Chaudhary P., Beniwal V., Sharma A.K. Microbial pigments as natural color sources: current trends and future perspectives. J Food Sci Technol. 2014;52:4669–4678. doi: 10.1007/s13197-014-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savvides A.L., Katsifas E.A., Hatzinikolaou D.G., Karagouni A. Xanthan production by Xanthomonas campestris using whey permeate medium. World J Microbiol Biotechnol. 2012;28:2759–2764. doi: 10.1007/s11274-012-1087-1. [DOI] [PubMed] [Google Scholar]
- 31.Kurbanoglu E.B., Ozdal M., Ozdal O.G., Algur O.F. Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. Braz J Microbiol. 2015;46:631–637. doi: 10.1590/S1517-838246246220131143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozdal M., Gürkök S., Ozdal O.G., Kurbanoğlu E.B. Rhamnolipid production by Pseudomonas aeruginosa OG1 using waste frying oil and ram horn peptone. AIP Conf Proc. 2017;1833:020102. doi: 10.1007/s13205-017-0774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Souw P., Demain A.L. Nutritional studies on xanthan production by Xanthomonas campestris NRRL B1459. Appl Environ Microbiol. 1979;37:1186–1192. doi: 10.1128/aem.37.6.1186-1192.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson I.W. Production of polysaccharide by Xanthomonas campestris in continuous culture. FEMS Microbiol Lett. 1978;3:347–349. [Google Scholar]
- 35.Candia J.L.F., Deckwer W.D. Effect of the nitrogen source on pyruvate content and rheological properties of xanthan. Biotechnol Prog. 1999;15:446–452. doi: 10.1021/bp990028i. [DOI] [PubMed] [Google Scholar]
- 36.Palaniraj A., Jayaraman V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J Food Eng. 2011;106:1–12. [Google Scholar]
- 37.Carignatto C.R.R., Oliveira K.S.M., de Lima V.M.G., de Oliva Neto P. New culture medium to xanthan production by Xanthomonas campestris pv. campestris. Indian J Microbiol. 2011;51:283–288. doi: 10.1007/s12088-011-0171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cadmus MC, Knutson CA Jr, U.S. Patent No. 4. Washington, DC: U.S. Patent and Trademark Office; 1983. 394,447.