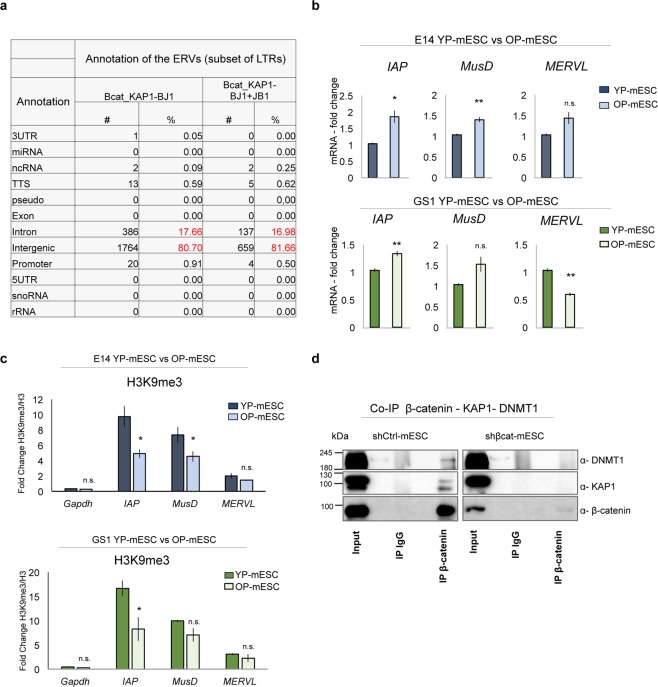
Figure 5.
β-catenin interacts with the chromatin repressive complex. (a) Table showing genomic annotation of common ERVs bound by β-catenin and KAP1 in BJ1 (columns 2, 3), and among β-catenin, KAP1 in BJ1 and KAP1 in JB1 mESCs (columns 4, 5), represented as number (#) and percentage (%) over the total common peaks. (b) Quantitative real-time PCR experiments showing the expression profiles of ERVs (IAP, MusD, MERVL) in E14 (upper charts) and GS1 (lower charts) YP- and OP- mESCs. The transcriptional levels are normalized to Gapdh as a reference gene. Data are represented as fold change (2−ΔΔCt) relative to the YP-mESCs and the results are means of n = 3 independent experiments ± SE. (c) ChIP-qPCR analysis of H3K9me3 recruitment at IAP, MusD and MERVL ERVs in E14 (upper charts) and GS1 (lower charts) YP- and OP- mESCs. The data are represented as fold change (2−ΔΔCt) over H3 and means of n = 3 independent experiments ± SE. (b,c) Asterisks indicate statistical significance calculated by unpaired two-tailed t test analysis (n.s. not significant; *p < 0.05; **p < 0.01). (d) Co-immunoprecipitation of β-catenin with either KAP1 or DNMT1 followed by western-blot analysis, in shCtrl -or shβcat- transduced E14 mESCs. 10% of input was used for DNMT1, KAP1 and β-catenin IP. IgG were used as negative control. An empty well was included between each experimental condition (Input, IgG and IP) to avoid cross-contamination.