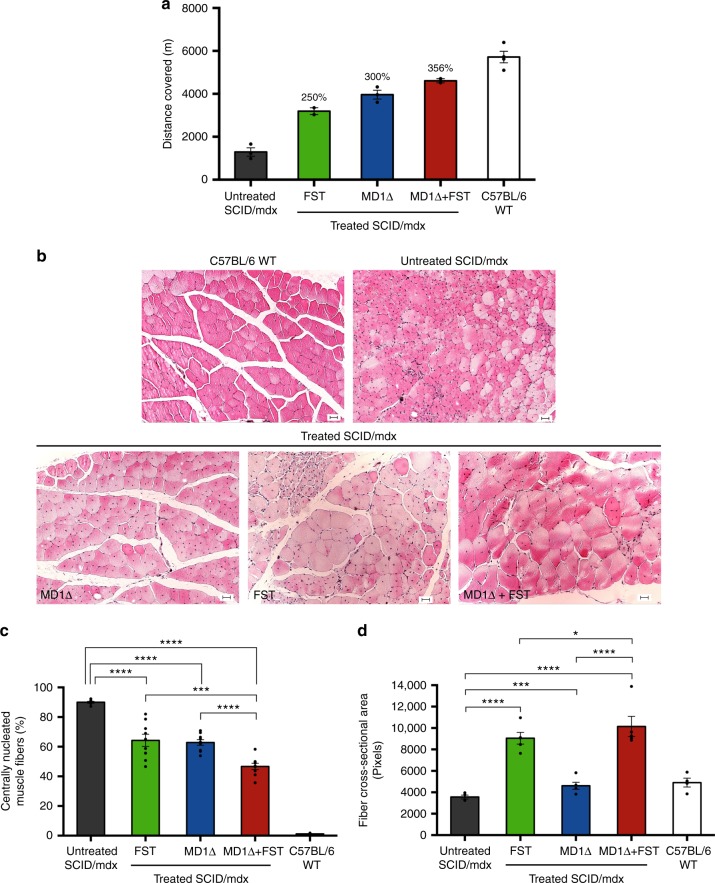
Fig. 6.
Phenotypic correction of muscular dystrophy in SCID/mdx mice. a SCID/mdx mice injected with ssAAV9-Sk-CRM4-Des-MD1Δ only, ssAAV9-Sk-CRM4-Des-FST only, and ssAAV9-Sk-CRM4-Des-MD1Δ with ssAAV9-Sk-CRM4-Des-FST combination therapy (2 × 1010 vg/mouse of each vector) were subjected to a treadmill assay. Physical performance of the 20-week-old SCID/mdx mice treated with the different therapeutic vectors compared to the untreated age-matched control SCID/mdx mice was determined by measuring the distance covered. Results were presented as mean ± standard error of the mean, (% increase in distance covered relative to untreated SCID/mdx was indicated. b–d Improvement of pathophysiological properties in SCID/mdx mice injected with ssAAV9-Sk-CRM4-Des-MD1Δ, ssAAV9-Sk-CRM4-Des-FST, ssAAV9-Sk-CRM4-Des-MD1Δ, and ssAAV9-Sk-CRM4-Des-FST combination therapy. b Hematoxylin and eosin staining of 5 μm thick transverse sections of the tibialis anterior muscle from mice treated with the different therapeutic vectors were compared with those of age-matched control C57BL/6 and SCID/mdx mice treated with PBS. The scale bars indicate 50 μm. c Graphical representation of the % of central nucleation in muscle fibers from treated sections versus the age-matched non-treated sections. Results were presented as mean ± standard error of the mean, ***p < 0.001 and ****p < 0.0001 using Student’s t-test (n = 4–9). d Muscle fiber cross-sectional area (expressed in pixels) of each of the different groups of SCID/mdx mice treated with the different therapeutic vectors (at a dose of 2 × 1010 vg/mouse). Untreated SCID/mdx and C57BL/6 mice were used as controls. Results were presented as mean ± standard error of the mean, *p < 0.05; ***p < 0.001, and ****p < 0.0001 using Student’s t-test (n = 4–5)