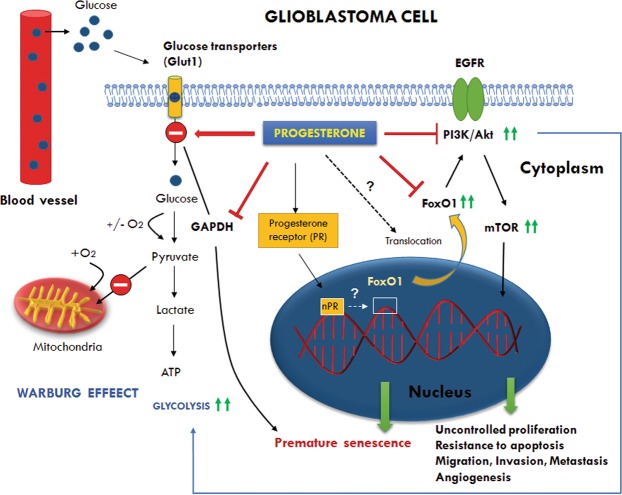
Figure 9.
Schematic representation of the modulatory effects of progesterone on glycolytic metabolism, PI3K/Akt/mTOR signaling, and FoxO1 transcription factor in GBM. The figure shows the preference for glycolysis over mitochondrial oxidation despite sufficient levels of oxygen (the Warburg effect) which supports high anabolic activity of cancer cells for uncontrolled proliferation, migration, invasion and metastasis. Glut1 (Glucose transporter isoform 1) facilitates glucose transport into the cytoplasm and its upregulation is observed in different cancers including GBM. GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) is a glycolysis enzyme whose overexpression is positively correlated with tumor progression in many cancers. Depletion of GAPDH is reported to induce senescence in tumor cells. FoxO1 (Forkhead box family O1) is a transcription factor which is critical for the regulation of cell cycle exit and arrest at G1, and induction of apoptosis. FoxO1 deregulation/inactivation leads to uncontrolled proliferation, and resistance to apoptosis. FoxO1 controls glycolytic metabolism through upstream PI3K/Akt/mTOR and downstream c-Myc activation. Our data showed that progesterone modulates glycolytic metabolism and induces premature senescence in GBM cells by inhibiting Glut1, GAPDH and cytoplasmic FoxO1 activity. Dotted arrows and question mark represent the modulatory effect of progesterone by an as yet unknown mechanism. How progesterone modulates nuclear FoxO1 activity or its translocation to the cytoplasm is still not known and needs to be defined.