Abstract
Background
Acute bronchiolitis is one of the most frequent causes of emergency department visits and hospitalisation in children. There is no specific treatment for bronchiolitis except for supportive treatment, which includes ensuring adequate hydration and oxygen supplementation. Continuous positive airway pressure (CPAP) aims to widen the lungs' peripheral airways, enabling deflation of overdistended lungs in bronchiolitis. Increased airway pressure also prevents the collapse of poorly supported peripheral small airways during expiration. Observational studies report that CPAP is beneficial for children with acute bronchiolitis. This is an update of a review first published in 2015.
Objectives
To assess the efficacy and safety of CPAP compared to no CPAP or sham CPAP in infants and children up to three years of age with acute bronchiolitis.
Search methods
We conducted searches of CENTRAL (2017, Issue 12), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1946 to December, 2017), Embase (1974 to December 2017), CINAHL (1981 to December 2017), and LILACS (1982 to December 2017) in January 2018.
Selection criteria
We considered randomised controlled trials (RCTs), quasi‐RCTs, cross‐over RCTs, and cluster‐RCTs evaluating the effect of CPAP in children with acute bronchiolitis.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data using a structured pro forma, analysed data, and performed meta‐analyses.
Main results
We included three studies with a total of 122 children (62/60 in intervention/control arms) aged up to 12 months that investigated nasal CPAP compared with supportive (or "standard") therapy. We included one new trial (72 children) that contributed data to the assessment of respiratory rate and need for mechanical ventilation for this update. The included studies were single‐centre trials conducted in France, the UK, and India. Two studies were parallel‐group RCTs and one was a cross‐over RCT. The evidence provided by the included studies was low quality; we assessed high risk of bias for blinding, incomplete outcome data, and selective reporting, and confidence intervals were wide.
The effect of CPAP on the need for mechanical ventilation in children with acute bronchiolitis was uncertain due to imprecision around the effect estimate (3 RCTs, 122 children; risk ratio (RR) 0.69, 95% confidence interval (CI) 0.14 to 3.36; low‐quality evidence). None of the trials measured time to recovery. Limited, low‐quality evidence indicated that CPAP decreased respiratory rate (2 RCTs, 91 children; mean difference (MD) ‐3.81, 95% CI ‐5.78 to ‐1.84). Only one trial measured change in arterial oxygen saturation, and the results were imprecise (19 children; MD ‐1.70%, 95% CI ‐3.76 to 0.36). The effect of CPAP on change in arterial partial carbon dioxide pressure (pCO₂) was imprecise (2 RCTs, 50 children; MD ‐2.62 mmHg, 95% CI ‐5.29 to 0.05; low‐quality evidence). Duration of hospital stay was similar in both CPAP and supportive care groups (2 RCTs, 50 children; MD 0.07 days, 95% CI ‐0.36 to 0.50; low‐quality evidence). Two studies did not report about pneumothorax, but pneumothorax did not occur in one study. No studies reported occurrences of deaths. Several outcomes (change in partial oxygen pressure, hospital admission rate (from emergency department to hospital), duration of emergency department stay, and need for intensive care unit admission) were not reported in the included studies.
Authors' conclusions
Limited, low‐quality evidence suggests that breathing improved (a decreased respiratory rate) in children with bronchiolitis who received CPAP; this finding is unchanged from the 2015 review. Further evidence for this outcome was provided by the inclusion of a low‐quality study for the 2018 update. Due to the limited available evidence, the effect of CPAP in children with acute bronchiolitis is uncertain for other outcomes. Larger, adequately powered trials are needed to evaluate the effect of CPAP for children with acute bronchiolitis.
Plain language summary
Continuous positive airway pressure (CPAP) for acute bronchiolitis in children
Review question
Is continuous positive airway pressure (CPAP) better or worse than supportive treatment for children with acute bronchiolitis?
Background
Bronchiolitis is inflammation of the small airways in the lungs, and a common cause for emergency department treatment among young children. Children usually receive supportive care that includes ensuring adequate hydration and providing supplementary oxygen as needed. Continuous positive airway pressure treatment involves providing positive air pressure by blowing air from a pump to keep airways open, and may be effective for children with bronchiolitis. This is an update of a review first published in 2015.
Search date
10 January 2018.
Study characteristics
We included three small randomised controlled trials (studies in which participants are assigned to one of two or more treatment groups using a random method) involving a total of 122 children aged up to 12 months who were diagnosed with bronchiolitis. We included one new low‐quality trial with 72 children in this update. The three studies were conducted at single centres in France, the UK, and India. All studies compared CPAP with standard therapy.
Study funding sources
One study was funded by a university hospital; one reported that no funding was received; and one did not mention the funding source.
Key results
Insufficient evidence was available to permit conclusions about the effect of CPAP on the need for mechanical ventilation in children with bronchiolitis. Limited, low‐quality evidence indicated that breathing improved (respiratory rate decreased) in children who received CPAP. The length of time children spent in hospital was similar between the CPAP and the standard therapy groups. No children in the studies were reported to have died. The studies did not report on time to recovery, change in partial oxygen pressure, how often children were admitted to hospital from the emergency department, how long children were in the emergency department, and the need for intensive care admission. There were no local nasal effects, or shock as reported by one study. No children were reported to have had air in the cavity between the lungs and the chest wall, causing lung collapse (pneumothorax) as reported by one study. Two studies did not report about local nasal effects, shock, or pneumothorax. The study added for this update contributed data to the assessment of respiratory rate and need for mechanical ventilation.
Quality of the evidence
We found limited, low‐quality evidence related to CPAP for children with bronchiolitis. Evidence quality was reduced due to high risk of bias, losses to follow‐up, selective reporting, and the wide range of values reported by the included studies.
Summary of findings
Summary of findings for the main comparison. Continuous positive airway pressure (CPAP) compared to supportive treatment for acute bronchiolitis in children.
| Continuous positive airway pressure (CPAP) compared to supportive treatment for acute bronchiolitis in children | ||||||
| Patient or population: children with acute bronchiolitis Settings: inpatient Intervention: CPAP Comparison: supportive treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Supportive treatment | CPAP | |||||
| Proportion of children requiring mechanical ventilation observation Follow‐up: 5 to 10 days | 50 per 1000 | 32 per 1000 | RR 0.69 (0.14 to 3.36) | 122 (3 studies) | ⊕⊕⊝⊝ low1,2 | |
| Time to recovery (hours) | Study population | Not estimable | 0 (0 studies) |
Not estimable | Data for time to recovery were not reported. | |
| Change in respiratory rate | The mean change in respiratory rate in the intervention groups was 3.81 lower (5.78 to 1.84 lower). | Mean change ‐3.81 (‐5.78 to ‐1.84) | 91 (2 studies) | ⊕⊕⊝⊝ low3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by one level due to high risk of bias in one study, participants lost to follow‐up, and selective reporting (Thia 2008). 2Wide confidence interval (Lal 2018; Thia 2008). 3Downgraded by one level due to high risk of bias in two domains (blinding of participants and personnel and blinding of outcome assessment) (Lal 2018).
Background
Description of the condition
Acute bronchiolitis is a frequent cause of emergency department visits and hospitalisation among infants (Hasegawa 2014; Praznik 2018; Rivera‐Sepulveda 2017). Bronchiolitis, an inflammation of the small airways of the lungs, is predominantly a viral disease, usually affecting infants and children aged up to three years. The most common cause is respiratory syncytial virus (RSV) (CDC 2018). In Italy, the hospitalisation rate reported for all infants with bronchiolitis aged up to one year was 5.4% (Lanari 2015). In the USA, hospitalisation rates varied from 1.7 to 2.1 per 100 infant‐seasons. 'Infant‐season' is defined as the number of children multiplied by the number of seasons, for example 50 infants seen in two seasons equates to 100 infant‐seasons. (Krilov 2017). Bronchiolitis occurs most frequently among non‐breastfed male infants who live in crowded conditions (Meates‐Dennis 2005).
Bronchiolitis typically presents with viral symptoms (sneezing, rhinorrhoea, and fever), which gradually progress to paroxysmal cough, wheezing, respiratory distress, and irritability. Chest findings are non‐specific and include wheezing, with or without fine crackles. Although not required for diagnosis, chest x‐ray may reveal hyperinflated lungs with patchy atelectasis. About 5% to 6% of children hospitalised with bronchiolitis respond poorly to treatment, and require intensive care management (Oakley 2017). About 75% of children admitted to intensive care units (ICU) require ventilatory support, and 18% of children requiring ventilatory support need invasive mechanical ventilation (Oakley 2017). Although uncommon, bronchiolitis may cause death; mortality rates range from 0.5% to 2% (Kabir 2003; Levy 1997). Mortality is higher in low‐income countries (Scheltema 2017). Improved intensive care support has significantly reduced bronchiolitis‐related mortality (Oakley 2017).
The standard management of bronchiolitis involves supportive care such as ensuring adequate fluid intake, antipyretics, and humidified oxygen supplementation if hypoxia is present (Davison 2004). Nebulised adrenaline, Hartling 2011a; Hartling 2011b, and hypertonic nebulised saline, Zhang 2017, have been found to be beneficial in acute bronchiolitis. Other therapeutic options, such as corticosteroids (Fernandes 2013), antibiotics (Farley 2014), bronchodilators (Gadomski 2014), heliox inhalation therapy (Liet 2015), chest physiotherapy (Roqué i Figuls 2016), nebulised recombinant human deoxyribonuclease (Merkus 2001; Nasr 2001), and steam inhalation (Umoren 2011), have been tried with no definitive benefit in bronchiolitis. A recent network meta‐analysis of interventions for bronchiolitis found that epinephrine plus corticosteroids and epinephrine plus hypertonic saline were more effective than placebo (Guo 2018).
Description of the intervention
Continuous positive airway pressure (CPAP) keeps airways open by administering positive pressure to the airways of spontaneously breathing patients throughout the respiratory cycle (Gupta 2016). Continuous positive airway pressure may be given to infants using nasal prongs, nasopharyngeal tube, or an infant nasal mask. Continuous positive airway pressure is administered using a commercially available circuit used in conjunction with a continuous flow source, or a ventilator. Continuous positive airway pressure devices may include provision of heated and humidified airflow. Continuous positive airway pressure use has been associated with adverse effects, including local and systemic effects, such as nasal mucosal damage, nasal excoriation, scarring, pressure necrosis, and nasal septum distortion (Gupta 2016; Lee 2002; Robertson 1996), aspiration secondary to gastric insufflation (Kiciman 1998), pneumothorax (de Bie 2002), and decreased cardiac output due to impaired pulmonary blood flow (Lee 2002).
How the intervention might work
The peripheral airways are most severely affected by inflammation in people with bronchiolitis. In infants with acute bronchiolitis, expiratory resistance is greater than inspiratory resistance, suggesting dynamic narrowing of the airways on expiration (Bont 2009). Acute bronchiolitis is associated with increased thoracic gas volume (air trapping) and total pulmonary resistance, with decreased dynamic compliance (Bont 2009). Infants initially compensate for the increased physiological dead space by increased respiratory rate, resulting in increased minute ventilation. Infants gradually become exhausted, and minute ventilation falls with increase in partial pressure of carbon dioxide (pCO₂) and hypoxaemia. From this point, the infant may improve with oxygen supplementation, or may progress to respiratory failure.
Continuous positive airway pressure increases the functional residual capacity of lungs, which results in enlargement of the diameter of almost all airways, including the peripheral airways. The widening of the peripheral airways enables deflation of overdistended lungs in bronchiolitis. Increased airway pressure also prevents the collapse of poorly supported peripheral small airways during expiration. Continuous positive airway pressure has been used for people with bronchiolitis, and benefits have been reported in observational studies (Soong 1993). Continuous positive airway pressure may prevent the need for mechanical ventilation in infants with acute bronchiolitis.
Why it is important to do this review
Acute bronchiolitis is a common clinical condition affecting infants and young children, yet no specific treatment is available except for supportive therapy. Continuous positive airway pressure is often used in the management of bronchiolitis on an empirical basis (i.e. based on personal experience without good evidence from the literature). We aimed to assess the role of CPAP for children with bronchiolitis.
Objectives
To assess the efficacy and safety of CPAP compared to no CPAP or sham CPAP in infants and children up to three years of age with acute bronchiolitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), quasi‐RCTs, cross‐over RCTs, and cluster‐RCTs were eligible for inclusion.
Types of participants
Children aged up to three years with a clinical diagnosis of acute bronchiolitis. We included all infants, regardless of respiratory syncytial virus (RSV) status.
Types of interventions
We included CPAP treatment with any pressure level, and delivered by any type of device, by any mode (nasal prongs, face mask, etc.), and for any duration, compared to no CPAP or sham CPAP. We included studies that applied CPAP at any time after patient presentation. We excluded studies that investigated the use of high‐flow nasal cannulae; this concept is addressed in another Cochrane Review (Beggs 2014). We included trials in which all children who were randomised to treatment and control arms received similar management in all other respects.
Types of outcome measures
Primary outcomes
Proportion of children requiring mechanical ventilation.
Time to recovery (as defined by the study).
Secondary outcomes
Change in respiratory rate.
Change in arterial oxygen saturation.
Change in arterial partial pressure of carbon dioxide (pCO₂) and partial pressure of oxygen (pO₂).
Hospital admission rate (from emergency department to hospital).
Duration of emergency department stay.
Duration of hospital stay.
Need for intensive care unit admission.
Adverse events, e.g. local nasal effects, pneumothorax, and shock.
Mortality.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 12), which includes the Cochrane Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (1946 to December, 2017), Embase (1974 to December 2017), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1981 to December 2017), and LILACS (Latin American and Caribbean Health Science Information database) (1982 to December 2017) on 10 January 2018.
We used the search strategy in Appendix 1 to search CENTRAL and MEDLINE. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase (Appendix 2), CINAHL (Appendix 3), and LILACS (Appendix 4). We did not apply any date, language, or publication restrictions.
Searching other resources
We searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) for completed and ongoing trials on 16 January 2018. We reviewed the reference lists of included studies to identify any additional studies. We contacted corresponding authors of included trials to ask about any additional ongoing RCTs or unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (KRJ, JLM) independently assessed the titles and abstracts of studies obtained by the search to identify potentially relevant studies. We retrieved the full text of potentially relevant studies, and two review authors (KRJ, JLM) independently assessed these for inclusion in the review. A review author (KRJ) corresponded with study authors to clarify study eligibility where necessary. We listed excluded studies with the reasons for their exclusion. Any disagreements were resolved by discussion.
Data extraction and management
Two review authors (KRJ, JLM) independently extracted data using a predefined data collection form in accordance with guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Data extracted included: source, eligibility, methods, participants and settings, interventions, outcomes, results, adverse effects, study funding source, and potential conflicts of interest. Any disagreements were resolved by discussion.
Assessment of risk of bias in included studies
Two review authors (KRJ, JLM) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion. We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided quotes from the study report together with a justification for our judgement in the 'Risk of bias' tables. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
We took risk of bias into account for studies that contributed to a given outcome when considering treatment effects.
Assessment of bias in conducting the systematic review
We conducted the review according to a published protocol and reported deviations from it in Differences between protocol and review.
Measures of treatment effect
We entered outcome data for each study into data tables in Review Manager 5 to calculate treatment effects (Review Manager 2014). We calculated risk ratio (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes.
We undertook meta‐analyses only where this was meaningful, that is where the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
Unit of analysis issues
We planned to include RCTs, quasi‐RCTs, cross‐over RCTs, and cluster‐RCTs. We considered data from the first study period for meta‐analysis from cross‐over trials. We plan that if we include cluster‐RCTs in future review updates, we will conduct meta‐analyses using the generic inverse‐variance method in Review Manager 5 (Review Manager 2014). We plan to add standard parallel‐group trials to the same generic inverse‐variance meta‐analysis.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
If numerical outcome data such as standard deviations or correlation coefficients were missing and could not be obtained from the study authors, these were calculated from other available statistics, such as P values, according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Where possible, we extracted data to enable intention‐to‐treat analysis, which aims to include all participants randomised into a trial irrespective of what happened subsequently. We calculated and reported losses to follow‐up if there was a discrepancy in the numbers randomised and the numbers analysed in each treatment group.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity before pooling. We compared inclusion and exclusion criteria among the included studies to assess clinical heterogeneity. We assessed statistical heterogeneity by looking at forest plots, using a Chi² test and the I² statistic. Using the Chi² test, a low P value of < 0.1 (or a large Chi² test statistic relative to its degree of freedom) provided evidence of heterogeneity of intervention effects. We interpreted the value of the I² statistic as follows:
0% to 40%, heterogeneity might not be important;
> 40% to 60%, moderate heterogeneity;
> 60% to 80%, substantial heterogeneity; and
> 80% to 100%, considerable heterogeneity.
Assessment of reporting biases
We planned that if we were able to pool more than 10 trials, we would create and examine funnel plots to explore possible small‐study and publication biases.
Data synthesis
We carried out meta‐analyses using Review Manager 5 (Review Manager 2014). We used a fixed‐effect model for pooled data analysis. We used a random‐effects meta‐analysis if there was important (more than 40%) statistical heterogeneity among studies.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table presenting the following outcomes: proportion of children requiring mechanical ventilation; time to recovery; and change in respiratory rate. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro 2015). We justified all decisions to downgrade the quality of evidence in footnotes, and made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for the following groups.
CPAP with oxygen and CPAP with heliox.
RSV‐positive and RSV‐negative children.
Different CPAP pressure levels (< 6 cm, 6 cm to 10 cm, and > 10 cm water level (H₂O)).
CPAP method: nasal prongs or face mask.
CPAP duration (< 12 hours, 12 to 24 hours, > 24 hours).
Trials with no CPAP and sham CPAP as comparator.
RCTs and cross‐over RCTs.
We planned to use the Chi² test to test for subgroup interactions in Review Manager 5 (Review Manager 2014).
Sensitivity analysis
We planned to perform sensitivity analyses to test the robustness of the results.
Repeating the meta‐analysis after excluding studies with inadequate allocation concealment.
Repeating the meta‐analysis after excluding studies in which the outcome evaluation was not blinded.
Repeating the meta‐analysis imputing missing data as best‐possible and worst‐possible outcomes.
Comparing the difference in pooled analysis results by using fixed‐effect and random‐effects models.
Results
Description of studies
We based all results on published data. We contacted study authors to request further details but received no additional information.
Results of the search
We have presented search results for this update only. Searches in January 2018 identified 102 records (Figure 1). After removing duplicates, we screened 65 records by title and abstract. We retrieved four full‐text reports for assessment, of which three studies were excluded (Cesar 2017; Chidini 2015; Milési 2017); see Characteristics of excluded studies; Figure 1. We included one new study for this update (Lal 2018).
1.
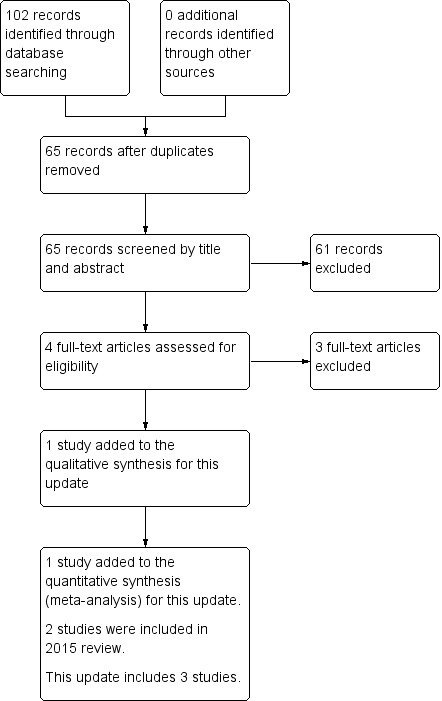
Study flow diagram for review update.
Included studies
We included three studies providing data from 122 children (Lal 2018; Milési 2013; Thia 2008); see Characteristics of included studies).
Design
All included studies were single‐centre trials (Lal 2018; Milési 2013; Thia 2008). Lal 2018 was described as a prospective, open‐label randomised trial; Milési 2013 as a prospective, parallel‐group RCT; and Thia 2008 was a randomised cross‐over study.
Sample sizes
The three included studies presented data from a total of 122 children. Sample sizes ranged from 19 children (10/9 treatment/control) in Milési 2013, to 72 children (36/36 treatment/control) in Lal 2018. We only included data from children in the first phase of the cross‐over RCT (31 children; 16/15 treatment/control) by Thia 2008.
Setting
The included studies were single‐centre trials conducted in India (Lal 2018), France (Milési 2013), and the UK (Thia 2008). Two trials involved children who were inpatients (Lal 2018; Thia 2008). The study by Milési 2013 involved children being treated in a paediatric intensive care unit (PICU).
Participants
Children's ages
Lal 2018 included children clinically diagnosed with bronchiolitis, but their age range was not clearly defined. Milési 2013 included infants aged up to six months, and Thia 2008 enrolled children aged up to 12 months. The mean age of children in the CPAP group was 6.8 ± 0.9 weeks and 10.92 ± 41.33 weeks in the trials by Milési 2013 and Thia 2008, respectively. The respective mean ages of children in the control groups were 8.2 ± 1.7 weeks and 10.5 ± 48.93 weeks (Milési 2013; Thia 2008).
Children's sex
Only Lal 2018 reported children's sex (N = 72): there were 26 boys and 10 girls in the treatment group, and 28 boys and 8 girls in the control group.
Respiratory syncytial virus (RSV) status
Milési 2013 included only children with RSV‐positive bronchiolitis. Thia 2008 included 20 (of 31) children with RSV‐positive bronchiolitis. Lal 2018 did not report RSV status.
Study inclusion criteria
Lal 2018 included hospitalised children with a diagnosis of acute bronchiolitis. Milési 2013 included children with severe respiratory distress defined by a modified Wood's clinical asthma score (m‐WCAS) > 4; no invasive or non‐invasive ventilation, including nasal continuous positive airway pressure (nCPAP), before admission to PICU. Thia 2008 enrolled children with capillary pCO₂ measurements > 6 kPa.
None of the children in the three included trials were reported to have comorbidities.
Study exclusion criteria
Lal 2018 excluded children in imminent need of mechanical ventilation. Milési 2013 excluded children with underlying cardiopulmonary or neuromuscular disease and who had pneumothorax on chest radiograph. Thia 2008 excluded children with congenital heart disease, neuromuscular disease, or mid‐face dysmorphism that prohibited the use of nasal prongs, those who required immediate invasive ventilation, and pCO₂ > 12 kPa.
Interventions
Lal 2018 used bubble CPAP delivered in the children's ward with a Gregory circuit; the pressure generated was not reported. Bubble CPAP treatment was provided for one hour once only. Children in the control arm received "standard care" in the form of adequate hydration and oxygen support through mask or hood for one hour once only. Children in the treatment group in the Milési 2013 trial received nCPAP 6 cm H₂O delivered using the Infant Flow Ventilator via a mask connected to a twin injector nozzle fixed to the child using a specially designed bonnet for children for six hours. Children in the control group received a heated and humidified air/oxygen mixture delivered through a nasal cannula that allowed a maximum gas flow of 2.5 L/min. (Milési 2013). Children in the treatment group in the study by Thia 2008 received "standard treatment" (defined as minimal handling, intravenous fluids, and oxygen by nasal prongs or face mask) plus nCPAP for 12 hours followed by standard treatment alone for the next 12 hours. Children in the control group received standard treatment alone for 12 hours followed by standard treatment plus nCPAP for the next 12 hours.
Outcomes
The primary outcome in the study by Lal 2018 was change in respiratory rate after the first hour of treatment. The secondary outcome was change in Silverman‐Anderson score, and a Modified Paediatric Society of New Zealand Severity Score (MPSNZ‐SS) before starting treatment and at one hour following the start of treatment (Lal 2018).
The primary outcome in Milési 2013 was clinical score for respiratory distress at baseline and at six hours after beginning treatment. Respiratory distress was evaluated with the m‐WCAS. Secondary outcome measures were respiratory and cardiac rate, average blood pressure at baseline and six hours. However, the outcome measures for this study were not clearly defined in the published study report, and data were taken from information at ClinicalTrials.gov. Other secondary outcome measures were manometric: variation of oesophageal pressure at baseline and 6 hours, and gasometric: minimal fraction of inspired oxygen (FiO₂) necessary to reach an oxygen saturation between 94% and 98%, transcutaneous pCO₂, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO₂/FiO₂) (Milési 2013).
Thia 2008 assessed change in pCO₂ at 12 hours of intervention as the primary outcome measure. Secondary outcomes were capillary pH, respiratory rate, pulse rate, and the need for invasive ventilatory support (Thia 2008).
Funding sources
Lal 2018 reported that no funding was received; Milési 2013 was funded by the clinical research department of a university hospital; and Thia 2008 did not mention source of funding.
Excluded studies
We excluded nine studies (Characteristics of excluded studies). Three studies were not RCTs (Balanzat 2006; Javouhey 2008; Smith 1993); two studies investigated comparisons that were not relevant to this review (Cesar 2017; Milési 2017); and four studies investigated interventions that were not relevant to this review (Chidini 2011; Chidini 2015; Hough 2011; Yañez 2008). We excluded three studies for this update (Cesar 2017; Chidini 2015; Milési 2017).
Studies awaiting classification
We did not identify any studies awaiting classification.
Ongoing studies
We did not identify any ongoing studies.
Risk of bias in included studies
'Risk of bias' assessments are presented in Figure 2 and Figure 3.
2.
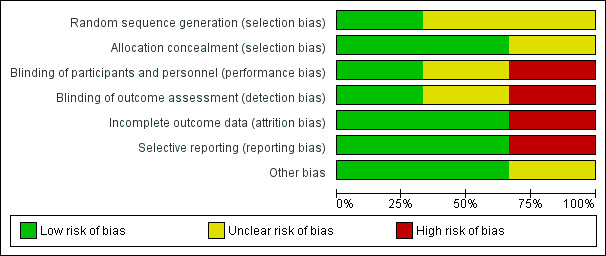
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
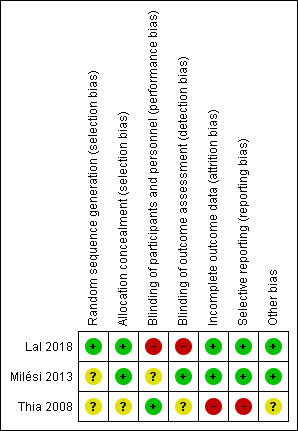
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
We assessed the method of random sequence generation as at unclear risk of bias due to inadequate reporting in two included studies (Milési 2013; Thia 2008). Lal 2018 used a computer software package for sequence generation and was assessed as at low risk of bias. We assessed allocation concealment as at low risk of bias in two studies that used sequentially numbered, opaque, and sealed envelopes (Lal 2018; Milési 2013). The method of allocation concealment was not reported for Thia 2008 and was therefore assessed as at unclear risk of bias.
Blinding
We assessed blinding of parents of included infants as at high risk of bias for the study by Lal 2018, which was an open‐label study. Milési 2013 did not report blinding and was assessed as at unclear risk of bias. We assessed Thia 2008 as at low risk of bias for this domain.
Incomplete outcome data
Lal 2018 reported that four children in the treatment arm (two who required mechanical ventilation and two did not tolerate CPAP) and one child who required mechanical ventilation in the control arm did not complete the intervention; we assessed this study as at low risk of bias because data for all randomised children were included in the analysis. Milési 2013 reported no losses to follow‐up and was assessed as at low risk of bias. Thia 2008 reported that two children from the control arm (one who required mechanical ventilation and another who was re‐allocated to the CPAP arm) did not complete the intervention and were not included in the analysis. We assessed this study as at high risk of bias for this domain (Thia 2008).
Selective reporting
We assessed Lal 2018 as at low risk of bias for selective reporting, as all prespecified outcomes were reported. Milési 2013 reported all prespecified outcomes and was assessed as at low risk of bias for this domain. Thia 2008 did not report data for a secondary outcome (capillary pH) and was assessed as at high risk of bias for this domain.
Other potential sources of bias
The included studies were free of other potential sources of bias.
Effects of interventions
See: Table 1
We based all results on published data. We contacted study authors to request further details but received no additional information.
Primary outcomes
1. Proportion of children requiring mechanical ventilation
In Lal 2018, two children in the CPAP group (N = 36) and one child in the control group (N = 36) required ventilation. No children in Milési 2013 required mechanical ventilation. Thia 2008 reported that two children in the control group (N = 15) and no children in the CPAP group (N = 16) required mechanical ventilation. The difference was not statistically significant (risk ratio 0.69, 95% confidence interval (CI) 0.14 to 3.36; 122 children; 3 studies; I² = 34%; Analysis 1.1; Figure 4; low‐quality evidence).
1.1. Analysis.
Comparison 1 Proportion of children requiring mechanical ventilation, Outcome 1 Proportion of children requiring mechanical ventilation.
4.
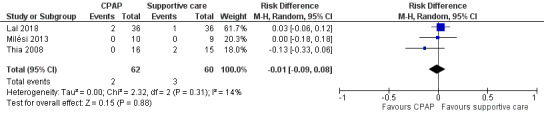
Forest plot of comparison: 1.1 Proportion of children requiring mechanical ventilation.
2. Time to recovery (as defined by the included trials)
None of the included studies provided data regarding children's time to recovery.
Secondary outcomes
1. Change in respiratory rate
Data for change in respiratory rate from start to end of intervention were available from two studies (Lal 2018; Milési 2013). The respiratory rate was decreased in children in the CPAP group (mean difference (MD) ‐3.81, 95% CI ‐5.78 to ‐1.84; 91 children; 2 studies; I² = 34%; Analysis 2.1; Figure 5; low‐quality evidence). There was no significant change in respiratory rate between groups in the study by Thia 2008. However, numerical values were not provided, and data could not be pooled for meta‐analysis. Decreased respiratory rate is a beneficial effect for children with bronchiolitis.
2.1. Analysis.
Comparison 2 Clinical improvements, Outcome 1 Change in respiratory rate.
5.
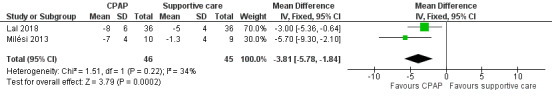
Forest plot of comparison: 2.1 Change in respiratory rate.
2. Change in arterial oxygen saturation
Only Milési 2013 provided data for this outcome. Change in arterial oxygen saturation did not differ between groups (MD ‐1.70, 95% CI ‐3.76 to 0.36; 19 children; I² = 0%; Analysis 2.2).
2.2. Analysis.
Comparison 2 Clinical improvements, Outcome 2 Change in arterial oxygen saturation.
3. Change in arterial partial pressure of carbon dioxide (pCO₂) and partial pressure of oxygen (pO₂)
Two studies provided data for this outcome (Milési 2013; Thia 2008). Change in pCO₂ did not differ between children in the CPAP group and those in the control group (MD ‐2.62, 95% CI ‐5.29 to 0.05; 50 children; 2 studies; I² = 15%; Analysis 2.3; Figure 6).
2.3. Analysis.
Comparison 2 Clinical improvements, Outcome 3 Change in arterial partial pressure of carbon dioxide (pCO₂) and partial pressure of oxygen (pO₂).
6.
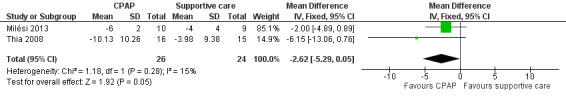
Forest plot of comparison: 2.3 Change in arterial partial pressure of carbon dioxide (mmHg).
4. Hospital admission rate (from emergency department to hospital)
None of the included trials reported this outcome.
5. Duration of emergency department stay
None of the included trials reported this outcome.
6. Duration of hospital stay
Two studies reported data for this outcome (Milési 2013; Thia 2008). Duration of hospital stay was similar between intervention and control groups (MD 0.07, 95% CI ‐0.36 to 0.50; 50 children; 2 studies; I² = 0%; Analysis 2.4).
2.4. Analysis.
Comparison 2 Clinical improvements, Outcome 4 Duration of hospital stay (days).
7. Need for intensive care unit admission
None of the included trials reported this outcome.
8. Adverse events, such as local nasal effects, pneumothorax, and shock
Two studies did not report data related to local nasal effects, pneumothorax, and shock (Milési 2013; Thia 2008). Lal 2018 reported no local nasal effects, pneumothorax, or shock in either group, although two children in the CPAP group had irritability. No other adverse events were reported.
9. Mortality
There were no deaths reported in the included studies.
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not possible because there were too few included studies for these to be conducted.
Discussion
Summary of main results
We evaluated the effects of CPAP for acute bronchiolitis in children. We included three studies that involved a total of 122 children. We found no difference in the requirement for mechanical ventilation among children treated with CPAP compared with those administered supportive care.
Limited, low‐quality evidence suggests the respiratory rate among children with bronchiolitis who receive CPAP is decreased. Change in arterial partial pressure of carbon dioxide (pCO₂) did not differ between children who received CPAP and those who received standard treatment. Hospital stay duration was similar between children treated with CPAP and those who received standard treatment. The included studies did not assess time to recovery, change in arterial oxygen saturation, change in partial pressure of oxygen (pO₂), hospital admission rate (from emergency department to hospital), duration of emergency department stay, or need for intensive care unit admission. Two included studies reported no events of pneumothorax or other adverse effects such as local nasal effects and shock. There were no local nasal effects, pneumothorax, and shock as reported by one study. Lal 2018 reported that two children in the CPAP group experienced irritability.
We assessed the quality of the evidence as low (Table 1).
Overall completeness and applicability of evidence
The three included studies were small (total of 122 children). To detect a 20% difference in the primary outcome (proportion of children requiring mechanical ventilation) with 80% power, 88 participants per group (total 176) are required. Data were not available for most of the secondary outcomes of this review. There was high risk of bias for two domains in one included trial (Thia 2008). We found some evidence to suggest that CPAP for acute bronchiolitis is beneficial in decreasing respiratory rate, but evidence was lacking for other outcomes.
Quality of the evidence
We found low‐quality evidence for both proportion of children requiring mechanical ventilation and change in respiratory rate (Table 1). We downgraded evidence quality because of high risk of bias for incomplete outcome data (participants lost to follow‐up) and selective reporting, and wide confidence intervals (Lal 2018; Thia 2008). We assessed evidence quality for change in respiratory rate as low due to high risk of bias in Lal 2018, which did not blind participants (parents of children), study personnel, or outcome assessors.
Potential biases in the review process
The search strategy for this review was broad and designed by the Cochrane Acute Respiratory Infections Information Specialist. It is unlikely that relevant studies were missed. Two review authors independently carried out study selection, data extraction, and analysis. There was no blinding of participants, study personnel, or outcome assessors, and this domain was assessed as at high risk of bias (see Characteristics of included studies). We assessed Milési 2013 as at unclear risk of bias for random sequence generation (method not reported) and blinding of participants and personnel (blinding not reported) (see Characteristics of included studies). We assessed Thia 2008 as at unclear risk of bias for random sequence generation (method not reported), allocation concealment (method not reported), and blinding of outcome assessment (not reported), and at high risk of bias for incomplete outcome data (two children did not complete the study and were not included in the analysis) and selective reporting (one outcome was not reported) (see Characteristics of included studies). Data were not available for many outcomes, and we were unable to obtain additional information from trial authors. Thia 2008 was a cross‐over trial, and we used data from the first trial phase for meta‐analysis, which may have decreased study power.
Agreements and disagreements with other studies or reviews
Donlan 2011 conducted a systematic review of CPAP use for acute bronchiolitis that included both randomised and observational studies. Only one study, Thia 2008, was common to both Donlan 2011 and our review. Donlan 2011 reported that CPAP reduced pCO₂, respiratory rate, and m‐WCAS in acute bronchiolitis, but assessed the quality of the evidence as low. Donlan 2011 also found no conclusive evidence that CPAP reduced need for intubation.
An excluded study, Chidini 2011, compared CPAP delivered by helmet versus facial mask. Chidini 2011 concluded that CPAP delivered by helmet was associated with more successful treatment outcomes, less sedation and sores, and a similar improvement in oxygenation with respect to the facial mask in cases of acute lung injury.
Another excluded study, Yañez 2008, compared non‐invasive ventilation using inspiratory positive airway pressure and expiratory positive airway pressure plus standard treatment (study group) to standard treatment (control group) in 50 children with acute respiratory failure. Non‐invasive ventilation was associated with improvement in hypoxaemia and the signs and symptoms of acute respiratory failure with protection from endotracheal intubation (Yañez 2008).
Observational studies have suggested that CPAP is beneficial for children with acute viral bronchiolitis (Cambonie 2008; Essouri 2011; Larrar 2006; McNamara 1997; Soong 1993).
Authors' conclusions
Implications for practice.
Several outcomes of this review were not measured in the included studies, and where data were available, findings were not sufficiently precise to enable drawing definitive conclusions for most outcomes. Limited, low‐quality evidence suggests a decreased respiratory rate among children with bronchiolitis who receive continuous positive airway pressure (CPAP), but there is a lack of evidence for other outcomes.
Implications for research.
Larger, adequately powered trials are needed to evaluate the effects of CPAP in children with acute bronchiolitis. The timing and duration of CPAP application, level of CPAP, type of device for CPAP application, and both clinical (including side effects, e.g. vomiting and aspiration) and laboratory outcomes need to be evaluated in future trials.
What's new
| Date | Event | Description |
|---|---|---|
| 10 January 2018 | New citation required but conclusions have not changed | The evidence for one secondary outcome (respiratory rate) was strengthened by the addition of a study with low‐quality evidence. Our conclusions remain unchanged. |
| 10 January 2018 | New search has been performed | We included one new trial, (Lal 2018), and excluded three trials (Cesar 2017; Chidini 2015; Milési 2017), for this update. |
Acknowledgements
We thank Sarah Thorning, formerly Information Specialist with Cochrane Acute Respiratory Infections, for developing the electronic search strategy for the 2015 review. We would also like to acknowledge Liz Dooley, Managing Editor of Cochrane Acute Respiratory Infections, for her support. We also thank the following people for commenting on the draft review: Edward Grandi, Rodrigo Cavallazzi, Anne Greenough, Teresa Neeman, and Lubna Al‐Ansary.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1 exp Bronchiolitis/ 2 bronchiolit*.tw. 3 Bronchopneumonia/ 4 bronchopneumon*.tw. 5 exp respiratory syncytial viruses/ or exp respiratory syncytial virus, human/ 6 Respiratory Syncytial Virus Infections/ 7 (respiratory syncytial virus* or rsv).tw. 8 or/1‐7 9 Respiratory Therapy/ 10 Respiration, Artificial/ 11 positive‐pressure respiration/ or continuous positive airway pressure/ 12 (positive pressur* adj5 (ventilat* or respir* or breath* or airway*)).tw. 13 positiv* airway* pressur*.tw. 14 continuous distend* pressur*.tw. 15 positive end expiratory pressure.tw. 16 (ppv or cpap or ncpap or nm‐cpap or np‐cpap or peep).tw. 17 or/9‐16 18 8 and 17
Appendix 2. Embase (Elsevier) search strategy
#14 *'continuous distending pressure'*:ab,ti 46* #13 *ppv*:ab,ti OR *cpap*:ab,ti OR *ncpap*:ab,ti OR *'nm‐cpap'*:ab,ti OR *'np‐cpap'*:ab,ti 17000* #12 *'positive airway pressure'*:ab,ti 6991* #11 ((*'positive pressure'* OR *'positive‐pressure'*) NEAR/5 (*ventilat** OR *respir** OR *breath** OR *airway**)):ab,ti 5253* #10 *'artificial ventilation'*/de OR *'positive end expiratory pressure'*/de OR *'cpap device'*/de 78111* #9 *#1* OR *#2* OR *#3* OR *#4* OR *#5* OR *#6* OR *#7* OR *#8**28466* #8 *rsv*:ab,ti 7727* #7 *'respiratory syncytial virus'*:ab,ti OR *'respiratory syncytial viruses'*:ab,ti 8252* #6 *'respiratory syncytial virus infection'*/de 1132* #5 *'respiratory syncytial pneumovirus'*/de 10173* #4 *bronchopneumon**:ab,ti 2426* #3 *'bronchopneumonia'*/de 3528* #2 *bronchiolit**:ab,ti 8269* #1 *'bronchiolitis'*/exp 11323*
Appendix 3. CINAHL (EBSCO) search strategy
S28 S18 AND S27 15 S27 S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 189,818 S26 (MH "Quantitative Studies") 8,622 S25 TI placebo* OR AB placebo* 20,274 S24 TI random* OR AB random* 100,950 S23 (MH "Random Assignment") 29,181 S22 TI ((singl* or doubl* or tripl* or trebl*) N1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) N1 (blind* or mask*)) 14,743 S21 TI clinic* N1 trial* OR AB clinic* N1 trial* 28,628 S20 PT clinical trial 50,141 S19 (MH "Clinical Trials+") 113,828 S18 S9 AND S17 89 S17 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 14,880 S16 TI (ppv or cpap or ncpap or nm‐cpap or np‐cpap or peep) OR AB (ppv or cpap or ncpap or nm‐cpap or np‐cpap or peep) 1,882 S15 TI positive end expiratory pressur* OR AB positive end expiratory pressur* 627 S14 TI continuous distending pressure OR AB continuous distending pressure 7 S13 TI positive airway* pressur* OR AB positive airway* pressur* 1,306 S12 TI ( (positive‐pressure or positive pressure) N5 (ventilat* or respir* or breath* or airway*) ) OR AB ( (positive‐pressure or positive pressure) N5 (ventilat* or respir* or breath* or airway*) ) 2,199 S11 (MH "Positive Pressure Ventilation") OR (MH "Continuous Positive Airway Pressure") OR (MH "Positive End‐Expiratory Pressure") 4,063 S10 (MH "Respiratory Therapy") OR (MH "Respiration, Artificial") 10,565 S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 1,924 S8 TI rsv OR AB rsv 478 S7 TI respiratory syncytial virus* OR AB respiratory syncytial virus*693 S6 (MH "Respiratory Syncytial Virus Infections") 784 S5 (MH "Respiratory Syncytial Viruses") 283 S4 TI bronchopneumon* OR AB bronchopneumon* 43 S3 (MH "Bronchopneumonia") 40 S2 TI bronchiolit* OR AB bronchiolit* 748 S1 (MH "Bronchiolitis+") 692
Appendix 4. LILACS (BIREME) search strategy
(mh:bronchiolitis OR bronchiolit* OR bronquiolitis OR bronquiolite OR mh:c08.127.446.135* OR mh:c08.381.495.146.135* OR mh:c08.730.099.135* OR mh:bronchopneumonia OR bronchopneumon* OR bronconeumonía OR broncopneumonia OR mh:"Respiratory Syncytial Viruses" OR "Virus Sincitiales Respiratorios" OR "Vírus Sinciciais Respiratórios" OR "Virus Sincitial Respiratorio" OR "Virus Sincicial Respiratorio" OR "Virus Sinciciales Respiratorios" OR "Vírus Sincicial Respiratório" OR mh:"Respiratory Syncytial Virus, Human" OR "Virus Sincitial Respiratorio Humano" OR "Vírus Sincicial Respiratório Humano" OR mh:"Respiratory Syncytial Virus Infections" OR "Infecciones por Virus Sincitial Respiratorio" OR "Infecções por Vírus Respiratório Sincicial" OR "respiratory syncytial virus" OR "respiratory syncytial viruses" OR rsv) AND (mh:"Respiratory Therapy" OR "Terapia Respiratoria" OR mh:"Respiration, Artificial" OR "Respiración Artificial" OR "Respiração Artificial" OR mh:"Positive‐Pressure Respiration" OR "Respiración con Presión Positiva" OR "Respiração com Pressão Positiva" OR "Positive End‐Expiratory Pressure" OR mh:"Continuous Positive Airway Pressure" OR "Presión de las Vías Aéreas Positiva Contínua" OR "Pressão Positiva Contínua nas Vias Aéreas" OR "Airway Pressure Release Ventilation" OR "Ventilación Liberadora de Presión de las Vías Aéreas" OR "Pressão Positiva Contínua nas Vias Respiratórias" OR "Ventilação com Liberação de Pressão das Vias Aéreas" OR vlpva OR "Ventilação com Liberação de Pressão das Vias Respiratórias" OR "positive airway pressure" OR "continuous distending pressure" OR ppv OR cpap OR ncpap OR "nm‐cpap" OR "np‐cpap" OR peep OR "positive pressure ventilation" OR "positive pressure respiration" OR "positive pressure breathing") AND db:("LILACS") AND type_of_study:("clinical_trials")
Data and analyses
Comparison 1. Proportion of children requiring mechanical ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of children requiring mechanical ventilation | 3 | 122 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.09, 0.08] |
Comparison 2. Clinical improvements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in respiratory rate | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐3.81 [‐5.78, ‐1.84] |
| 2 Change in arterial oxygen saturation | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐1.7 [‐3.76, 0.36] |
| 3 Change in arterial partial pressure of carbon dioxide (pCO₂) and partial pressure of oxygen (pO₂) | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.62 [‐5.29, 0.05] |
| 4 Duration of hospital stay (days) | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.36, 0.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lal 2018.
| Methods | Study design: prospective, open‐label, randomised, single‐centre study Study duration: November 2014 to March 2016 |
|
| Participants |
Inclusion criteria
Study enrolment criteria: 72 children hospitalised with clinical diagnosis of acute bronchiolitis were eligible for inclusion in the study. Bronchiolitis was defined as respiratory distress (respiratory rate ≥ 50/min) in an infant aged from 1 month to 1 year, along with wheezing on auscultation and hyperinflated lung. Exclusion criteria: infants who were in imminent need of ventilator support were excluded. |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
Primary outcomes: change in respiratory rate after the first hour of treatment Secondary outcomes: change in Silverman‐Anderson score, and a Modified Paediatric Society of New Zealand Severity Score (MPSNZ‐SS): before starting treatment, and at 1 hour following the start of treatment Need for mechanical ventilation was reported. Time to recovery was not reported. |
|
| Notes | Funding source: no funding received Contact with study authors for additional information: yes. However, no additional information was provided. Other: conflict of interest stated as none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks of 8 using computer software |
| Allocation concealment (selection bias) | Low risk | Allocation done using sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 child in the control arm (who needed mechanical ventilation) and 4 children in the intervention arm (2 who needed mechanical ventilation and 2 who did not tolerate CPAP) did not complete the study. Intention‐to‐treat analysis conducted. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in study were reported. |
| Other bias | Low risk | Funding: declared by authors as none Competing interests: declared by authors as none |
Milési 2013.
| Methods | Study design: prospective, randomised, single‐centre study Duration of study: November 2006 to March 2009 |
|
| Participants |
Inclusion criteria
Study enrolment criteria
|
|
| Interventions | Active intervention: nCPAP 6 cm H₂O with the Infant Flow Ventilator via a mask connected to a twin injector nozzle fixed to the child by a specially designed bonnet for 6 hours Control: infants in the control group continued to receive a heated and humidified air/oxygen mixture delivered through a nasal cannula, which allowed a maximum gas flow of 2.5 L/min | |
| Outcomes |
Primary outcomes: clinical score for respiratory distress at baseline and at 6 hours after beginning the procedure. Respiratory distress was evaluated with m‐WCAS.
Secondary outcomes: respiratory and cardiac rate, average blood pressure at baseline and 6 hours Note: outcome measures were not clearly defined in the published trial; these were taken from the registered trial at ClinicalTrials.gov Manometric: variation of oesophageal pressure at baseline and 6 hours Gasometric: minimal FiO₂ necessary to reach an oxygen saturation between 94% and 98%, transcutaneous pCO₂, PaO₂/FiO₂ |
|
| Notes | Funding source: Clinical Research Department of Montpellier University Hospital Centre. The study was conducted at the Pediatric Intensive Care Unit, CHU Montpellier, France over 3 consecutive RSV epidemic periods, from November 2006 to March 2009. Contact with study authors for additional information: yes. However, no additional information was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation is not clearly reported. Children "were randomly assigned" to intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of the allocated intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All defined outcomes were reported. |
| Other bias | Low risk | Conflicts of interest: declared by authors as none Funding source: Clinical Research Department of Montpellier University Hospital Centre |
Thia 2008.
| Methods | Study design: randomised cross‐over, single‐centre study Duration of study: October 2002 to March 2005 |
|
| Participants |
Inclusion criteria
Study enrolment criteria: children aged up to 12 months with clinical diagnosis of bronchiolitis and capillary pCO₂ measurements > 6 kPa Exclusion criteria: children with congenital heart disease, neuromuscular disease, and mid‐face dysmorphism prohibiting the use of nasal prongs, requiring immediate invasive ventilation, and pCO₂ > 12 kPa were excluded |
|
| Interventions | Eligible children were randomised to receive either standard treatment plus nCPAP for 12 hours followed by standard treatment alone for the next 12 hours, or standard treatment alone for 12 hours followed by standard treatment plus nCPAP for the next 12 hours. Standard treatment was defined as minimal handling, intravenous fluids, and oxygen by nasal prongs or face mask. Nasal CPAP was applied using the Infant Flow System with pressures of 5 to 6 cm H₂O. | |
| Outcomes |
Primary outcome: change in pCO₂ at 12 hours of intervention Secondary outcomes: capillary pH, respiratory rate, pulse rate, and the need for invasive ventilatory support |
|
| Notes | The study was conducted over 3 winters from October 2002 to March 2005. Funding: not reported Contact with study authors for additional information: yes. However, no additional information was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind participants and personnel to the interventions due to the inherently different methods of administration. Unblinding was less likely to have introduced bias because the primary outcome was objective in nature. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 children (1 who required ventilation and another who shifted to CPAP) did not complete the intervention in control arm and were not included in the analysis. |
| Selective reporting (reporting bias) | High risk | A secondary outcome (capillary pH) mentioned in the study methods section was not reported. |
| Other bias | Unclear risk | Conflicts of interest: none Funding: not reported |
CPAP: continuous positive airway pressure FiO₂: fraction of inspired oxygen H₂O: pressure level measures as water column m‐WCAS: modified Wood's clinical asthma score nCPAP: nasal continuous positive airway pressure PaO₂/FiO₂: ratio of arterial partial pressure of oxygen to fraction of inspired oxygen pCO₂: arterial partial pressure of carbon dioxide pH: measurement unit for acidity PICU: paediatric intensive care unit RSV: respiratory syncytial virus SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balanzat 2006 | Not RCT. Observational study |
| Cesar 2017 | Wrong comparison. Study compared high‐flow nasal canula with CPAP. |
| Chidini 2011 | Wrong intervention. Study compared different methods of CPAP delivery rather than CPAP versus no CPAP. |
| Chidini 2015 | Wrong intervention. Study compared different methods of CPAP delivery rather than CPAP versus no CPAP. |
| Hough 2011 | Wrong intervention. Study compared different levels of CPAP produced by high‐flow nasal prongs. |
| Javouhey 2008 | Not RCT. Retrospective study |
| Milési 2017 | Wrong comparison. Study compared high‐flow nasal canula with CPAP. |
| Smith 1993 | Not RCT. Study compared different levels of peak end expiratory pressure in mechanically ventilated children with bronchiolitis. |
| Yañez 2008 | Wrong intervention. Study evaluated non‐invasive ventilation rather than CPAP. |
CPAP: continuous positive airway pressure RCT: randomised controlled trial
Differences between protocol and review
We included both the primary outcomes and change in respiratory rate in the 'Summary of findings' table. Sufficient data were not available for the outcomes of hospital admission rate and adverse events for inclusion in the 'Summary of findings' table as specified in the protocol.
Contributions of authors
KRJ and JLM: selected studies, independently extracted data, and approved the final version of the review. KRJ: performed analyses. JLM: critically reviewed the analysis and provided important intellectual input for the original review and review update.
Sources of support
Internal sources
Internet and library facility from Government Medical College Hospital, Chandigarh, India.
External sources
No sources of support supplied
Declarations of interest
Kana R Jat: none known Joseph L Mathew: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Lal 2018 {published data only}
- Lal SN, Kaur J, Anthwal P, Goyal K, Bahl P, Puliyel JM. Nasal continuous positive airway pressure in bronchiolitis: a randomized controlled trial. Indian Pediatrics 2018;55(1):27‐30. [PubMed] [Google Scholar]
Milési 2013 {published data only}
- Milési C, Matecki S, Jaber S, Mura T, Jacquot A, Pidoux O, et al. 6 cm H₂O continuous positive airway pressure versus conventional oxygen therapy in severe viral bronchiolitis: a randomized trial. Pediatric Pulmonology 2013;48(1):45‐51. [DOI] [PubMed] [Google Scholar]
Thia 2008 {published data only}
- Thia LP, McKenzie SA, Blyth TP, Minasian CC, Kozlowska WJ, Carr SB. Randomised controlled trial of nasal continuous positive airways pressure (CPAP) in bronchiolitis. Archives of Disease in Childhood 2008;93(1):45‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Balanzat 2006 {published data only}
- Balanzat AMC, Lazarte G, Surarez V, Bonilla ME, Gighi M, Chede C, et al. Effects of different levels of nasal continuous positive airways pressure (CPAP) in infants with severe acute bronchiolitis. European Respiratory Journal 2006;28(Suppl 50):P1531. [Google Scholar]
Cesar 2017 {published data only}
- Cesar R, Bispo B, Felix PH, Modolo MC, Cabo S, Souza A, et al. A randomized controlled trial of high‐flow nasal cannula versus CPAP in critical bronchiolitis. Critical Care Medicine 2017;46(1):553. [Google Scholar]
Chidini 2011 {published data only}
- Chidini G, Piastra M, Wolfler A, Marchesi T, Calderini E, Conti G, et al. Noninvasive continuous positive airway pressure (NCPAP) by helmet versus facial mask: a multicenter RCT. Intensive Care Medicine 2011;135(Suppl 2):331‐2. [Google Scholar]
Chidini 2015 {published data only}
- Chidini G, Piastra M, Marchesi T, Luca D, Napolitano L, Salvo I, et al. Continuous positive airway pressure with helmet versus mask in infants with bronchiolitis: an RCT. Pediatrics 2015;135(4):e868‐75. [DOI] [PubMed] [Google Scholar]
Hough 2011 {published data only}
- Hough JL, Pham TMT, Schibler A. Delivery of high flow nasal prong oxygen: the effect CPAP exposed. Pediatric Critical Care Medicine 2011;12(Suppl):A7. [Google Scholar]
Javouhey 2008 {published data only}
- Javouhey E, Barats A, Richard N, Stamm D, Floret D. Non‐invasive ventilation as primary ventilatory support for infants with severe bronchiolitis. Intensive Care Medicine 2008;34(9):1608‐14. [DOI] [PubMed] [Google Scholar]
Milési 2017 {published data only}
- Milési C, Essouri S, Pouyau R, Liet JM, Afanetti M, Portefaix A, et al. High flow nasal cannula (HFNC) versus nasal continuous positive airway pressure (nCPAP) for the initial respiratory management of acute viral bronchiolitis in young infants: a multicenter randomized controlled trial (TRAMONTANE study). Intensive Care Medicine 2017;43(2):209‐16. [DOI] [PubMed] [Google Scholar]
Smith 1993 {published data only}
- Smith PG, el‐Khatib MF, Carlo WA. PEEP does not improve pulmonary mechanics in infants with bronchiolitis. American Review of Respiratory Disease 1993;147(5):1295‐8. [DOI] [PubMed] [Google Scholar]
Yañez 2008 {published data only}
- Yañez LJ, Yunge M, Emilfork M, Lapadula M, Alcántara A, Fernández C, et al. A prospective, randomized, controlled trial of noninvasive ventilation in pediatric acute respiratory failure. Pediatric Critical Care Medicine 2008;9(5):484‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beggs 2014
- Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JE. High‐flow nasal cannula therapy for infants with bronchiolitis. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD009609.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bont 2009
- Bont L. Current concepts of the pathogenesis of RSV bronchiolitis. Advances in Experimental Medicine and Biology 2009;634:31‐40. [DOI] [PubMed] [Google Scholar]
Cambonie 2008
- Cambonie G, Milési C, Jaber S, Amsallem F, Barbotte E, Picaud JC, et al. Nasal continuous positive airway pressure decreases respiratory muscles overload in young infants with severe acute viral bronchiolitis. Intensive Care Medicine 2008;34(10):1865‐72. [DOI] [PubMed] [Google Scholar]
CDC 2018
- Centers for Disease Control and Prevention. Respiratory syncytial virus. www.cdc.gov/rsv/index.html (accessed prior to 21 January 2019).
Davison 2004
- Davison C, Ventre KM, Luchetti M, Randolph AG. Efficacy of interventions for bronchiolitis in critically ill infants: a systematic review and meta‐analysis. Pediatric Critical Care Medicine 2004;5(5):482‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Bie 2002
- Bie HMA, Toledo‐Epping L, Verbeke J, Elburg RM. Neonatal pneumatocele as a complication of nasal continuous positive airway pressure. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86(3):F202‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donlan 2011
- Donlan M, Fontela PS, Puligandla PS. Use of continuous positive airway pressure (CPAP) in acute viral bronchiolitis: a systematic review. Pediatric Pulmonology 2011;46(8):736‐46. [DOI] [PubMed] [Google Scholar]
Essouri 2011
- Essouri S, Durand P, Chevret L, Balu L, Devictor D, Fauroux B, et al. Optimal level of nasal continuous positive airway pressure in severe viral bronchiolitis. Intensive Care Medicine 2011;37(12):2002‐7. [DOI] [PubMed] [Google Scholar]
Farley 2014
- Farley R, Spurling GK, Eriksson L, Mar CB. Antibiotics for bronchiolitis in children under two years of age. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD005189.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandes 2013
- Fernandes RM, Bialy LM, Vandermeer B, Tjosvold L, Plint AC, Patel H, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD004878.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadomski 2014
- Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001266.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 28 June 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guo 2018
- Guo C, Sun X, Wang X, Guo Q, Chen D. Network meta‐analysis comparing the efficacy of therapeutic treatments for bronchiolitis in children. Journal of Parenteral and Enteral Nutrition 2018;42(1):186‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2016
- Gupta S, Donn SM. Continuous positive airway pressure: physiology and comparison of devices. Seminars in Fetal & Neonatal Medicine 2016;21(3):204‐11. [DOI] [PubMed] [Google Scholar]
Hartling 2011a
- Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD003123.pub3] [DOI] [PubMed] [Google Scholar]
Hartling 2011b
- Hartling L, Fernandes RM, Bialy L, Milne A, Johnson D, Plint A, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta‐analysis. BMJ 2011;342:d1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasegawa 2014
- Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Temporal trends in emergency department visits for bronchiolitis in the United States, 2006 to 2010. Pediatric Infectious Disease Journal 2014;33:11‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kabir 2003
- Kabir ML, Haq N, Hoque M, Ahmed F, Amin R, Hossain A, et al. Evaluation of hospitalized infants and young children with bronchiolitis ‐ a multi centre study. Mymensingh Medical Journal 2003;12(2):128‐33. [PubMed] [Google Scholar]
Kiciman 1998
- Kiciman NM, Andréasson B, Bernstein G, Mannino FL, Rich W, Henderson C, et al. Thoracoabdominal motion in newborns during ventilation delivered by endotracheal tube or nasal prongs. Pediatric Pulmonology 1998;25(3):175‐81. [DOI] [PubMed] [Google Scholar]
Krilov 2017
- Krilov LR, Fergie J, Goldstein M, McLaurin KK, Wade S, Diakun D, et al. National bronchiolitis hospitalization rates among preterm and full term infants: 2010–2015. Open Forum Infectious Disease 2017;4(Suppl 1):695. [Google Scholar]
Lanari 2015
- Lanari M, Prinelli F, Adorni F, Santo S, Vandini S, Silvestri M, et al. Risk factors for bronchiolitis hospitalization during the first year of life in a multicenter Italian birth cohort. Italian Journal of Pediatrics 2015;41:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larrar 2006
- Larrar S, Essouri S, Durand P, Chevret L, Haas V, ChabernaudJL, et al. Effects of nasal continuous positive airway pressure ventilation in infants with severe acute bronchiolitis. Archives of Pediatrics 2006;13(11):1397‐403. [DOI] [PubMed] [Google Scholar]
Lee 2002
- Lee SY, Lopez V. Physiological effects of two temperature settings in preterm infants on nasal continuous airway pressure ventilation. Journal of Clinical Nursing 2002;11(6):845‐7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Levy 1997
- Levy BT, Graber MA. Respiratory syncytial virus infection in infants and young children. Journal of Family Practice 1997;45(6):473‐81. [PubMed] [Google Scholar]
Liet 2015
- Liet JM, Ducruet T, Gupta V, Cambonie G. Heliox inhalation therapy for bronchiolitis in infants. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD006915.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNamara 1997
- McNamara F, Sullivan CE. Nasal CPAP treatment in an infant with respiratory syncytial virus‐associated apnea. Pediatric Pulmonology 1997;24(3):218‐21. [DOI] [PubMed] [Google Scholar]
Meates‐Dennis 2005
- Meates‐Dennis M. Bronchiolitis. Archives of Disease in Childhood. Education and Practice Edition 2005;90(4):ep81‐6. [Google Scholar]
Merkus 2001
- Merkus PJ, Hoog M, Gent R, Jongste JC. DNase treatment for atelectasis in infants with severe respiratory syncytial virus bronchiolitis. European Respiratory Journal 2001;18(4):734‐7. [PubMed] [Google Scholar]
Nasr 2001
- Nasr SZ, Strouse PJ, Soskolne E, Maxvold NJ, Garver KA, Rubin BK, et al. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest 2001;120(1):203‐8. [DOI] [PubMed] [Google Scholar]
Oakley 2017
- Oakley E, Chong V, Borland M, Neutze J, Phillips N, Krieser D, et al. Intensive care unit admissions and ventilation support in infants with bronchiolitis. Emergency Medicine Australasia 2017;29(4):421‐8. [DOI] [PubMed] [Google Scholar]
Praznik 2018
- Praznik A, Vinšek N, Prodan A, Erčulj V, Pokorn M, Mrvič T, et al. Risk factors for bronchiolitis severity: a retrospective review of patients admitted to the university hospital from central region of Slovenia. Influenza and Other Respiratory Viruses 2018;12(6):765‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivera‐Sepulveda 2017
- Rivera‐Sepulveda A, Garcia‐Rivera EJ. Epidemiology of bronchiolitis: a description of emergency department visits and hospitalizations in Puerto Rico, 2010‐2014. Tropical Medicine and Health 2017;45:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 1996
- Robertson NJ, McCarthy LS, Hamilton PA, Moss AL. Nasal deformities resulting from flow driver continuous positive airway pressure. Archives of Disease in Childhood 1996;75(3):F209‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roqué i Figuls 2016
- Roqué i Figuls M, Giné‐Garriga M, Granados RC, Perrotta C, Vilaró J. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD004873.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheltema 2017
- Scheltema NM, Gentile A, Lucion F, Nokes DJ, Munywoki PK, Madhi SA, et al. Global respiratory syncytial virus‐associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Global Health 2017;5(10):e984‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soong 1993
- Soong WJ, Hwang B, Tang RB. Continuous positive airway pressure by nasal prongs in bronchiolitis. Pediatric Pulmonology 1993;16(3):163‐6. [DOI] [PubMed] [Google Scholar]
Umoren 2011
- Umoren R, Odey F, Meremikwu MM. Steam inhalation or humidified oxygen for acute bronchiolitis in children up to three years of age. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD006435.pub2] [DOI] [PubMed] [Google Scholar]
Zhang 2017
- Zhang L, Mendoza‐Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2017, Issue 12. [DOI: 10.1002/14651858.CD006458.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Jat 2013
- Jat KR, Mathew JL. Continuous positive airway pressure (CPAP) for acute bronchiolitis in children. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010473] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jat 2015
- Jat KR, Mathew JL. Continuous positive airway pressure (CPAP) for acute bronchiolitis in children. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010473.pub2] [DOI] [PubMed] [Google Scholar]


