Abstract
Background
Ileus is common after gastrointestinal surgery and has been identified as a research priority. Several issues have limited previous research, including a widely accepted definition and agreed outcome measure. This review is the first stage in the development of a core outcome set for the return of bowel function after gastrointestinal surgery. It aims to characterize the extent of variation in current outcome reporting.
Methods
A systematic search of MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature) and the Cochrane Library was performed for 1990–2017. RCTs of adults undergoing gastrointestinal surgery, including at least one reported measure relating to return of bowel function, were eligible. Trial registries were searched across the same period for ongoing and completed (but not published) RCTs. Definitions of ileus and outcome measures describing the return of bowel function were extracted.
Results
Of 5670 manuscripts screened, 215 (reporting 217 RCTs) were eligible. Most RCTs involved patients undergoing colorectal surgery (161 of 217, 74·2 per cent). A total of 784 outcomes were identified across all published RCTs, comprising 73 measures (clinical: 63, 86 per cent; radiological: 6, 8 per cent; physiological: 4, 5 per cent). The most commonly reported outcome measure was ‘time to first passage of flatus’ (140 of 217, 64·5 per cent). The outcomes ‘ileus’ and ‘prolonged ileus’ were defined infrequently and variably.
Conclusion
Outcome reporting for the return of bowel function after gastrointestinal surgery is variable and not fit for purpose. An agreed core outcome set will improve the consistency, reliability and clinical value of future studies.
Introduction
Ileus, defined as a delayed return of bowel function after surgery, occurs in 10–20 per cent of patients undergoing elective gastrointestinal surgery, and may lead to painful abdominal distension, vomiting, constipation and distress1 2. As part of its research prioritization process, the Association of Coloproctology of Great Britain and Ireland identified ileus as a research priority, and called for further research to identify clinically effective, patient‐focused management strategies3.
Over the past 20 years, numerous interventions to optimize the return of bowel function have been tested in RCTs4. Many were limited by methodological challenges related to the assessment of complex interventions and heterogeneity in the choice of outcome measures5. Poor standardization and variation in the use of outcome measures is problematic for translation into clinical practice. Although several groups have attempted to address this problem through consensus statements, the impact of these efforts remains unclear6 7.
The COMET (Core Outcome Measures in Effectiveness Trials) Initiative aims to develop agreed ‘core outcome sets’, which represent the minimum information that should be reported in all trials of a specific condition8. No such outcomes set for the return of bowel function after gastrointestinal surgery has been developed. Consistent reporting would increase the value of RCTs, improve the quality of meta‐analyses and enhance decision‐making when integrating new treatments into clinical practice9.
This review represents the first stage in the development process. It aimed to identify all reported outcome measures for the return of bowel function used in RCTs of gastrointestinal surgery, as well as characterizing the extent of variation in order to develop an interim strategy for maximizing the value of future studies.
Methods
Study design
This study was developed with the assistance of patient representatives at a national stakeholder meeting in London, UK in April 2017. A systematic review was performed according to a predefined protocol. It was registered prospectively on the PROSPERO database of systematic reviews (CRD42017082351) and reported in line with the PRISMA guidelines10.
Search
A search strategy was developed to identify outcome measures used to assess the return of bowel function after gastrointestinal surgery (Table S1, supporting information). Two independent investigators performed searches of MEDLINE (via OvidSP), Embase (via OvidSP), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (via EBSCOhost) and the Cochrane Library on 15 June 2017. Titles and abstracts were screened for eligibility via Covidence Systematic Review Software (Veritas Health Innovation, Melbourne, Australia; www.covidence.org), followed by inspection of full‐text manuscripts as appropriate. Discrepancies between investigators were discussed with the review team until consensus was achieved. All ‘primary registries’ included in the WHO Registry Network (including ClinicalTrials.gov) were queried for ongoing and completed (but unpublished) RCTs (Table S2, supporting information). A simplified search strategy (ileus AND surgery) was applied to these databases. Unique trial identifiers and details of corresponding authors were used to de‐duplicate RCTs that were already identified as published manuscripts.
Eligibility criteria
All RCTs including adult patients (18 years old or above) undergoing gastrointestinal surgery were eligible for inclusion. Eligible studies had to include at least one outcome measure (primary or secondary) describing the return of bowel function or the incidence of ileus. RCTs published in a peer‐reviewed journal (in print or online) or registered on a WHO‐approved trials registry were included. Non‐randomized study designs, reports published in a non‐English language and grey literature were excluded.
Outcomes
The primary outcome was the number of outcome measures relating to the return of bowel function. Secondary outcomes were the type of outcome (primary or secondary) and mode of measurement (clinical, radiological or physiological). If outcomes of ‘ileus’ were reported, the definition assigned to ileus (or absence of) was documented.
Definitions
RCTs were defined as prospective studies with participants randomized to one of a minimum of two intervention groups. Gastrointestinal surgery was defined as any surgical procedure performed on the oesophagus, stomach, small bowel, colon, rectum or anus. The primary outcome of each RCT was identified according to information provided in the manuscript or trial registry entry, if available. Where ambiguity persisted, the primary outcome was identified according to the study's reported sample‐size calculation. Only outcomes describing the return of bowel function were considered; adverse events (such as nausea attributed directly to opioid consumption) and other generic measures of recovery (such as duration of hospital stay) were not considered. Outcome measures were classified as ‘clinical’ measures (those measured by clinicians or reported by patients), ‘physiological’ (those measuring physiological processes) and ‘radiological’ (those measured using either cross‐sectional or non‐cross‐sectional imaging). They were further classified according to core areas described by the OMERACT (Outcome Measures in Rheumatology) filter 2.0, including: Life Impact, Pathological Manifestations and Resource Use11. The core area of Death was not considered relevant in the present context. Individual outcome measures could be classified into one or multiple core areas.
Data extraction
A single investigator extracted data from eligible manuscripts. Data fields of interest included manuscript demographics (journal and year), procedure type and study characteristics (setting, population, recruitment and blinding). If data extraction was unclear, corresponding authors were contacted for clarification. Study outcome measures were extracted verbatim and grouped to produce frequencies. At this stage, small variations in reporting (such as ‘time to pass stool’ and ‘time taken to pass stool’) were rationalized.
Statistical analysis
Descriptive data are expressed using proportions and median values. A subgroup analysis of outcome measures for lower (colorectal) versus upper gastrointestinal surgery was planned prospectively. All data were entered into Microsoft Excel® (Microsoft, Redmond, Washington, USA) for analysis.
Bias assessment
As this review reported a summary description of outcome measures used in RCTs, rather than a measure of clinical data, assessments of bias for each RCT were not performed12 13.
Results
Study characteristics
A total of 5670 manuscripts were screened for eligibility, and 215 (reporting 217 RCTs) were included in the review (Fig. 1; Table S3, supporting information). Most RCTs involved patients undergoing colorectal surgery (161, 74·2 per cent), and the majority tested either a drug (100, 46·1 per cent) or a procedural (64, 29·5 per cent) intervention. Institutions in Asia (82 of 217, 37·8 per cent), Europe (70 of 217, 32·3 per cent) and North America (44 of 217, 20·3 per cent) were the most frequent study locations. The majority of RCTs were single‐centre studies (173, 79·7 per cent) reporting on small populations (fewer than 100 participants) (139, 64·1 per cent). The median number of outcomes relating to the return of bowel function was 3 (i.q.r. 2–5), and 82 RCTs (37·8 per cent) included one of these as a primary study outcome. Full study characteristics are shown in Table 1.
Figure 1.

Flow diagram of included studies. GI, gastrointestinal
Table 1.
Characteristics of the included RCTs
| No. of studies (n = 217) | |
|---|---|
| Year of publication | |
| 1990–1999 | 25 (11·5) |
| 2000–2009 | 85 (39·2) |
| 2010–2017 | 107 (49·3) |
| Type of surgery | |
| Colorectal | 161 (74·2) |
| Upper gastrointestinal | 29 (13·4) |
| Mixed | 27 (12·4) |
| Setting | |
| Africa | 2 (0·9) |
| Asia | 82 (37·8) |
| Australasia | 6 (2·8) |
| Europe | 70 (32·3) |
| North America | 44 (20·3) |
| South America | 4 (1·8) |
| Cross‐continental | 9 (4·1) |
| No. of centres | |
| 1 | 173 (79·7) |
| ≥ 2 | 44 (20·3) |
| No. of arms | |
| 2 | 186 (85·7) |
| 3 | 26 (12·0) |
| ≥ 4 | 5 (2·3) |
| Blinding | |
| None | 59 (27·2) |
| Single | 44 (20·3) |
| Double | 75 (34·6) |
| Unknown | 39 (18·0) |
| Recruitment | |
| < 100 | 139 (64·1) |
| ≥ 100 | 78 (35·9) |
| Intervention | |
| Behavioural | 3 (1·4) |
| Device | 3 (1·4) |
| Dietary supplement | 37 (17·1) |
| Drug | 100 (46·1) |
| Procedure | 64 (29·5) |
| Other | 10 (4·6) |
| No. of bowel recovery outcomes | |
| 1–5 | 169 (77·9) |
| 6–10 | 48 (22·1) |
| Bowel recovery as primary outcome | |
| Yes | 82 (37·8) |
| No | 103 (47·5) |
| Unclear | 32 (14·7) |
Values in parentheses are percentages.
Published outcome measures
Across all RCTs, a total of 784 outcomes relating to the return of bowel function were reported. These comprised 73 discrete outcome measures (Table 2). Approximately one‐third (25 of 73, 34 per cent) of identified outcome measures were reported only once without replication. Overall, the most frequently reported measures were ‘time to first passage of flatus’ (140 of 217, 64·5 per cent), ‘time to first passage of stool’ (69 of 217, 31·8 per cent) and ‘time to first bowel movement’ (65 of 217, 30·0 per cent). This differed slightly for the subset of RCTs involving upper gastrointestinal surgery, where the most common outcome measures were ‘time to first passage of flatus’ (20 of 29, 69 per cent), ‘need for nasogastric tube insertion/reinsertion’ (8 of 29, 28 per cent) and ‘time to first passage of stool’ (7 of 29, 24 per cent).
Table 2.
Reported outcomes in published RCTs
| Reported outcome measure | No. of reports | Mode of outcome | Primary outcome | Secondary outcome | Unclear outcome | ||
|---|---|---|---|---|---|---|---|
| Total | Colorectal* | UGI | |||||
| Time to first passage of flatus | 140 | 120 | 20 | Clinical | 19 (13·6) | 98 (70·0) | 23 (16·4) |
| Time to first passage of stool | 69 | 62 | 7 | Clinical | 18 (26) | 39 (57) | 12 (17) |
| Time to first bowel movement | 65 | 61 | 4 | Clinical | 16 (25) | 41 (63) | 8 (12) |
| Incidence of postoperative ileus | 58 | 52 | 6 | Clinical | 4 (7) | 44 (76) | 10 (17) |
| Need for nasogastric tube/replacement | 45 | 37 | 8 | Clinical | 2 (4) | 36 (80) | 7 (16) |
| Incidence and/or duration of vomiting | 38 | 35 | 3 | Clinical | 1 (3) | 30 (79) | 7 (18) |
| Time to first fluid intake | 28 | 25 | 3 | Clinical | 1 (4) | 23 (82) | 4 (14) |
| Time to first solid intake | 26 | 21 | 5 | Clinical | 0 (0) | 21 (81) | 5 (19) |
| Incidence and/or duration of nausea | 25 | 20 | 5 | Clinical | 0 (0) | 21 (84) | 4 (16) |
| Time to tolerate solid intake | 23 | 23 | 0 | Clinical | 1 (4) | 21 (91) | 1 (4) |
| Time to tolerate normal/regular diet | 22 | 21 | 1 | Clinical | 0 (0) | 21 (95) | 1 (5) |
| Time to detect bowel sounds | 21 | 19 | 2 | Clinical | 0 (0) | 14 (67) | 7 (33) |
| Time to first oral intake | 15 | 12 | 3 | Clinical | 0 (0) | 10 (67) | 5 (33) |
| GI‐2 composite outcome | 13 | 13 | 0 | Clinical | 3 (23) | 10 (77) | 0 (0) |
| Incidence of prolonged postoperative ileus | 13 | 13 | 0 | Clinical | 2 (15) | 11 (85) | 0 (0) |
| Incidence and/or extent of abdominal distension (generic scale) | 13 | 11 | 2 | Clinical | 0 (0) | 9 (69) | 4 (31) |
| Time to return of bowel function | 11 | 11 | 0 | Clinical | 0 (0) | 10 (90) | 1 (9) |
| Duration of nasogastric tube placement | 11 | 5 | 6 | Clinical | 0 (0) | 7 (64) | 4 (36) |
| Duration of postoperative ileus | 10 | 10 | 0 | Clinical | 1 (10) | 7 (70) | 2 (20) |
| GI‐3 composite outcome | 8 | 8 | 0 | Clinical | 6 (75) | 2 (25) | 0 (0) |
| Incidence and/or extent of abdominal pain (generic scale) | 8 | 7 | 1 | Clinical | 0 (0) | 8 (100) | 0 (0) |
| Gastrointestinal transit: number of remnant opaque markers (radiograph) | 7 | 6 | 1 | Radiological | 4 (57) | 3 (43) | 0 (0) |
| Proportion/amount of food intake per meal | 7 | 5 | 2 | Clinical | 0 (0) | 7 (100) | 0 (0) |
| Time to first soft food | 7 | 4 | 3 | Clinical | 0 (0) | 7 (100) | 0 (0) |
| Time to return of appetite | 6 | 5 | 1 | Clinical | 0 (0) | 3 (50) | 3 (50) |
| Readiness for discharge based on gastrointestinal function | 6 | 6 | 0 | Clinical | 0 (0) | 6 (100) | 0 (0) |
| Time to tolerate fluid intake | 5 | 5 | 0 | Clinical | 0 (0) | 5 (100) | 0 (0) |
| Time to tolerate oral intake | 5 | 5 | 0 | Clinical | 1 (20) | 3 (60) | 1 (20) |
| Gastrointestinal motility: gastric/duodenal pressure transducers (manometry) | 5 | 1 | 4 | Physiological | 3 (60) | 1 (20) | 1 (20) |
| Need for parenteral nutrition | 5 | 5 | 0 | Clinical | 0 (0) | 5 (100) | 0 (0) |
| Nutritional status: serum albumin | 4 | 1 | 3 | Clinical | 0 (0) | 4 (100) | 0 (0) |
| Volume of nasogastric tube aspirate | 3 | 2 | 1 | Clinical | 0 (0) | 2 (67) | 1 (33) |
| Need for antiemetic medication | 3 | 2 | 1 | Clinical | 0 (0) | 2 (67) | 1 (33) |
| Gastrointestinal transit: number of evacuated opaque markers (radiograph) | 3 | 3 | 0 | Radiological | 2 (67) | 1 (33) | 0 (0) |
| Duration of parenteral nutrition | 3 | 3 | 0 | Clinical | 0 (0) | 3 (100) | 0 (0) |
| Gastrointestinal transit: scintigraphy using standard meal | 3 | 3 | 0 | Radiological | 2 (67) | 1 (33) | 0 (0) |
| Time to intake of > 1000 ml fluid per day | 3 | 3 | 0 | Clinical | 0 (0) | 2 (67) | 1 (33) |
| Time to tolerate low‐residue diet | 2 | 2 | 0 | Clinical | 1 (50) | 1 (50) | 0 (0) |
| Incidence and/or duration of oral intolerance | 2 | 2 | 0 | Clinical | 1 (50) | 1 (50) | 0 (0) |
| Gastrointestinal QoL (Gastrointestinal Symptom Rating Scale) | 2 | 0 | 2 | Clinical | 0 (0) | 2 (100) | 0 (0) |
| Gastrointestinal transit: ultrasonography using standard meal | 2 | 2 | 0 | Radiological | 0 (0) | 1 (50) | 1 (50) |
| Frequency of stool | 2 | 1 | 1 | Clinical | 0 (0) | 1 (50) | 1 (50) |
| Time to first sips of water | 2 | 1 | 1 | Clinical | 0 (0) | 2 (100) | 0 (0) |
| Need for laxative medication | 2 | 2 | 0 | Clinical | 0 (0) | 2 (100) | 0 (0) |
| Incidence of readmission due to postoperative ileus | 2 | 2 | 0 | Clinical | 0 (0) | 1 (50) | 1 (50) |
| Gastrointestinal motility: intraoperative count of bowel peristaltic waves over 1 min | 2 | 1 | 1 | Clinical | 2 (100) | 0 (0) | 0 (0) |
| Gastric emptying: paracetamol (acetaminophen) absorption | 2 | 2 | 0 | Physiological | 1 (50) | 0 (0) | 1 (50) |
| Incidence and/or extent of upper abdominal pain (Visick scale) | 2 | 0 | 2 | Clinical | 0 (0) | 0 (0) | 2 (100) |
| Duration of prolonged postoperative ileus | 1 | 1 | 0 | Clinical | 1 (100) | 0 (0) | 0 (0) |
| Abdominal circumference ratio | 1 | 0 | 1 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Time to first stoma output | 1 | 1 | 0 | Clinical | 0 (0) | 0 (0) | 1 (100) |
| Incidence of morbidity due to postoperative ileus | 1 | 1 | 0 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Incidence of gastric upset | 1 | 1 | 0 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Incidence and/or extent of hunger (Visick scale) | 1 | 0 | 1 | Clinical | 0 (0) | 0 (0) | 1 (100) |
| Incidence of nasogastric tube aspirate > 500 ml per day | 1 | 0 | 1 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Time to second passage of flatus | 1 | 1 | 0 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Cumulative frequency of flatus (within defined time period) | 1 | 1 | 0 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Quantification of bowel gas (according to Gas Volume Score) | 1 | 0 | 1 | Radiological | 0 (0) | 1 (100) | 0 (0) |
| Time to first postoperative abdominal peristalsis | 1 | 1 | 0 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Incidence of early postoperative ileus | 1 | 1 | 0 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Incidence of late postoperative ileus | 1 | 1 | 0 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Time to occurrence of postoperative ileus | 1 | 0 | 1 | Clinical | 1 (100) | 0 (0) | 0 (0) |
| Frequency of bowel sounds | 1 | 1 | 0 | Clinical | 0 (0) | 0 (0) | 1 (100) |
| Incidence and/or extent of upper abdominal fullness (Visick scale) | 1 | 0 | 1 | Clinical | 0 (0) | 0 (0) | 1 (100) |
| Vomiting after nasogastric tube removal | 1 | 0 | 1 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Incidence of hiccups | 1 | 1 | 0 | Clinical | 0 (0) | 0 (0) | 1 (100) |
| Incidence and/or duration of belching | 1 | 1 | 0 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Incidence and/or duration of regurgitation | 1 | 0 | 1 | Clinical | 0 (0) | 0 (0) | 1 (100) |
| Incidence and/or duration of satiety | 1 | 1 | 0 | Clinical | 0 (0) | 1 (100) | 0 (0) |
| Consistency of stool (Bristol Stool Chart) | 1 | 0 | 1 | Clinical | 0 (0) | 0 (0) | 1 (100) |
| Gastrointestinal transit: scintigraphy (HIDA) – bile transit through small bowel | 1 | 0 | 1 | Radiological | 0 (0) | 1 (100) | 0 (0) |
| Gastrointestinal motility: electrogastroenterography | 1 | 1 | 0 | Physiological | 0 (0) | 1 (100) | 0 (0) |
| Gastric emptying: acetate breath test | 1 | 1 | 0 | Physiological | 0 (0) | 1 (100) | 0 (0) |
Values in parentheses are percentages.
Number of reports in colorectal studies (including studies of mixed colorectal and upper gastrointestinal tract (UGI)). GI‐2, composite outcome measure – time taken to pass first stool and tolerate oral intake; GI‐3, composite outcome measure – time taken to pass first stool or flatus and tolerate oral intake; QoL, quality of life; HIDA, hepatobiliary iminodiacetic acid.
Planned outcome measures
Some 96 eligible registry entries were identified (Fig. 1). Assessment of these entries identified 224 planned outcomes, comprising 36 discrete outcome measures relating to the return of bowel function (Table 3). The median number per trial was 2 (i.q.r. 1–3). The most commonly planned outcome measures were similar to those in published manuscripts, including ‘time to first passage of flatus’ (44 of 96, 46 per cent), ‘time to first passage of stool’ (30 of 96, 31 per cent) and ‘incidence of postoperative ileus’ (28 of 96, 29 per cent). Two unique outcome measures were identified, including ‘Gastrointestinal Quality of Life Index (GIQLI) score’ (1 of 96, 1 per cent) and ‘Gastrointestinal motility: dynamic MRI’ (1 of 96, 1 per cent). These were not replicated elsewhere.
Table 3.
Planned outcome measures in trial register entries
| Reported outcome measure | Mode of outcome | No. of reports (n = 96) |
|---|---|---|
| Time to first passage of flatus | Clinical | 44 (46) |
| Time to first passage of stool | Clinical | 30 (31) |
| Incidence of postoperative ileus | Clinical | 28 (29) |
| Time to first bowel movement | Clinical | 18 (19) |
| Time to tolerate solid intake | Clinical | 16 (17) |
| GI‐2 composite outcome | Clinical | 12 (13) |
| Incidence and/or duration of vomiting | Clinical | 12 (13) |
| Need for nasogastric tube/replacement | Clinical | 7 (7) |
| Incidence and/or duration of nausea | Clinical | 6 (6) |
| Time to return of bowel function | Clinical | 5 (5) |
| Time to detect bowel sounds | Clinical | 4 (4) |
| Incidence of prolonged postoperative ileus | Clinical | 4 (4) |
| GI‐3 composite outcome | Clinical | 3 (3) |
| Time to tolerate normal/regular diet | Clinical | 3 (3) |
| Time to tolerate low‐residue diet | Clinical | 3 (3) |
| Time to return of appetite | Clinical | 3 (3) |
| Duration of postoperative ileus | Clinical | 2 (2) |
| Incidence and/or extent of abdominal pain (generic scale) | Clinical | 2 (2) |
| Gastrointestinal transit: number of remnant SITZMARKS® markers (radiograph) | Radiological | 2 (2) |
| Volume of nasogastric tube aspirate | Clinical | 2 (2) |
| Time to tolerate fluid intake | Clinical | 2 (2) |
| Duration of prolonged postoperative ileus | Clinical | 2 (2) |
| Time to first solid intake | Clinical | 1 (1) |
| Time to first oral intake | Clinical | 1 (1) |
| Time to first fluid intake | Clinical | 1 (1) |
| Duration of nasogastric tube placement | Clinical | 1 (1) |
| Readiness for discharge based on gastrointestinal function | Clinical | 1 (1) |
| Time to tolerate oral intake | Clinical | 1 (1) |
| Gastrointestinal transit: number of evacuated SITZMARKS® markers (radiograph) | Radiological | 1 (1) |
| Incidence and/or duration of oral intolerance | Clinical | 1 (1) |
| Abdominal circumference ratio | Clinical | 1 (1) |
| Time to first stoma output | Clinical | 1 (1) |
| Incidence of morbidity due to postoperative ileus | Clinical | 1 (1) |
| Incidence of gastric upset | Clinical | 1 (1) |
| Gastrointestinal Quality of Life Index (GIQLI) score | Clinical | 1 (1) |
| Gastrointestinal motility: dynamic MRI | Radiological | 1 (1) |
Values in parentheses are percentages. SITZMARKS® (Konsyl Pharmaceuticals, Easton, Maryland, USA). GI‐2, composite outcome measure – time taken to pass first stool and tolerate oral intake; GI‐3, composite outcome measure – time taken to pass first stool or flatus and tolerate oral intake.
Classification of outcome measures
Across published and planned RCTs, clinical outcome measures (including clinical scales, instruments and consultations) were largely favoured: 63 of 73 (86 per cent) and 33 of 36 (92 per cent) respectively (Fig. 2). Radiological modalities comprised six of 73 (8 per cent) published measures and three of 36 (8 per cent) planned measures in trial registries. These included radiographic, nuclear and MRI assessments of gastrointestinal transit. Physiological measures were infrequent (4 of 73 (5 per cent) and 0 of 36 (0 per cent) respectively), and included measures of gastric emptying such as acetate breath‐testing, paracetamol (acetaminophen) absorption and gastric manometry. The vast majority of outcome measures were relevant to Life Impact and Pathological Manifestation core areas, with few concerned with Resource Use.
Figure 2.
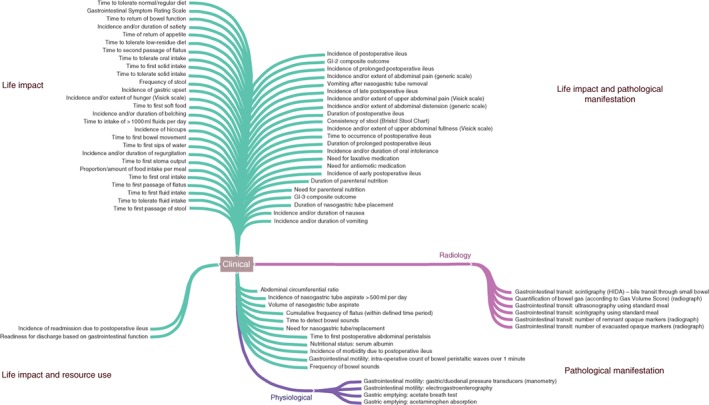
Map of 73 identified outcome measures according to core areas. GI‐2, composite outcome measure – time taken to pass first stool and tolerate oral intake; GI‐3, composite outcome measure – time taken to pass first stool or flatus and tolerate oral intake
Definitions of ileus
The outcome measures postoperative ileus and prolonged postoperative ileus were defined in 17 of 58 reports (29 per cent) and their duration was described in five of 13 reports (38 per cent). All reported definitions demonstrated considerable variability (Table 4). Most incorporated elements of both upper and lower gastrointestinal function (7 of 17 (41 per cent) and 4 of 5 (80 per cent) respectively), followed by elements of lower gastrointestinal function alone (6 of 17 (35 per cent) and 1 of 5 (20 per cent)). The majority of definitions defined postoperative ileus or prolonged postoperative ileus according to incidence (12 of 17 (71 per cent) and 4 of 5 (80 per cent)), whereas the remainder defined them according to the time taken to resolve (5 of 17 (29 per cent) and 1 of 5 (20 per cent)).
Table 4.
Definitions of ileus as reported in published literature
| Definition: incidence/duration of ‘postoperative ileus’ (n = 17 of 58, 29 per cent) |
|
‘delay of the first postoperative flatus lasting for >72 hours (3·0 days) after surgery… or some other status requiring intervention for treatment for ileus’ ‘Patients meeting 2 or more of the following 5 criteria on or after day 4 postoperatively – nausea OR vomiting over the preceding 12 hours; inability to tolerate a solid or semi‐solid oral diet over the preceding 2 meal times; abdominal distension; absence of flatus AND stool over the preceding 24 hours; and radiologic evidence of ileus on abdominal plain film or CT over the preceding 24 hours’ ‘defined as lack of passage of flatus or stool and intolerance to oral intake for at least 24 h… on day 5 after operation’ ‘intolerance to oral food in the absence of clinical and radiological data of mechanical obstruction for more than 72 hours, or the need for a nasogastric tube’ ‘Ileus persisted until patient had normal bowel sounds or he/she was passing flatus’ ‘defined as failure of bowel sounds to return within 12 h postoperatively’ ‘the number of days before signs of restoration of the bowel function appeared (bowel movement, flatus, and stool) after which oral intake was restarted’ ‘resolution defined as passage of flatus or stool in the absence of nausea, vomiting, or abdominal distention’ ‘need for nasogastric tube reinsertion or discontinuation of oral intake’ ‘Adynamic or paralytic ileus that persisted for > 3 days following surgery’ ‘the condition in which the time required for the resumption of flatus passage after surgery exceeded 48 hours’ ‘The resolution of postoperative ileus was defined as having a bowel movement in the absence of abdominal distention and vomiting’ ‘the interval between the end of surgery and the first passage of gas or stool through the anus’ ‘defined as postoperative nausea/vomiting, accompanied by abdominal distention, absence of bowel function, and x‐ray findings consistent with ileus’ ‘non‐passage of gas or solid per rectum for 5 days, cessation of such passage, vomiting (on day 1), nasogastric intubation, continued intravenous fluids’ ‘abdominal distension, no flatus or bowel movement, or nausea/vomiting that prevents oral intake or requires therapeutic use of nasogastric tube’ ‘the inability to tolerate and resume oral solid diet intake beyond 6 days after surgery’ |
| Definition: incidence/duration of ‘prolonged postoperative ileus’ (n = 5 of 13, 38 per cent) |
|
‘The presence of two or more of the following signs/symptoms over the 4th postoperative day: abdominal distension, persistent and nonspecific abdominal pain, vomits and/or nausea, the absence of passage of flatus or stools, oral intolerance, and radiological findings on plain radiology’ ‘Defined as being resolved when all 4 of the following criteria were met – absence of nausea AND vomiting for 12 hours with nasogastric tube spigotted or removed; ability to tolerate a solid or semi‐solid diet at the preceding mealtime; absence of abdominal distension; and passage of flatus OR stool over the preceding 24 hours’ ‘no passage of flatus or stool by the end of the fifth post‐operative day’ ‘the inability to tolerate diet 5 days after surgery in the absence of active bowel sounds with the need for nasogastric decompression’ |
| ‘Two or more of the following criteria: (i) nausea/vomiting, (ii) inability to tolerate an oral diet for > 24 h, (iii) absence of flatus for > 24 h, (iv) distension and (v) radiological confirmation occurring on or after day 4 postoperatively without prior resolution of POI’ |
Discussion
This review identified multiple outcome measures describing the return of bowel function after gastrointestinal surgery. There was consistency in reporting of a small number of terms, such as ‘time to first passage of flatus’ and ‘time to first passage of stool’, but one‐third of all measures were used only once without replication. The return of bowel function was often reported using multiple outcomes, which may have led to inconsistent results and unclear conclusions. When ileus was reported as an outcome, a corresponding definition was frequently not given; when this was provided, definitions varied considerably.
Previous research on ileus has been limited by several methodological challenges, including the lack of an agreed definition5, such as the wide variation in definitions for prolonged ileus in studies of colorectal surgery14. Several attempts have been made to overcome these difficulties through consensus agreements. A systematic review and global survey6 proposed a series of definitions for postoperative ileus, prolonged postoperative ileus and recurrent ileus to delineate broad patterns of bowel dysfunction. An international Delphi process7 found consensus on the importance of abdominal pain, distension and bowel sounds for identifying ileus in clinical practice. The role of bowel sounds was later discredited as a reliable measure15. Other efforts have included scoring tools to measure the clinical impact of delayed return of bowel function16. Some groups attempted to validate composite outcome measures such as the composite measure GI‐2 (defined as time to tolerance of oral diet and passage of stool) with good results (area under the curve 0·9)17. Currently, this offers the most representative assessment of return of gastrointestinal function after surgery, whereas the validity of other commonly used outcome measures remains unclear. Clinicians are increasingly recognizing the return of bowel function as a continuous spectrum, rather than a dichotomous outcome, ranging from transient nausea and vomiting to severe bowel dysmotility18.
Measuring the return of bowel function is important for clinicians and those responsible for planning and commissioning surgical services. For surgeons, it is important as a means of reducing postoperative complications. Previous research19 has suggested that early identification of delayed bowel function (followed by targeted interventions such as nasogastric decompression) may decrease the incidence of pulmonary complications. In the absence of consistent outcome measures, undertaking reliable meta‐analysis will remain difficult or simply impossible. For those involved in the organization of surgical services, it is important to have reliable evaluations of new treatments in order to plan the provision of efficient clinical services. Agreement on a core outcome set may offer an effective solution.
Based on this review, researchers should rationalize the number of outcome measures relating to the return of bowel function until an agreed core outcome set exists. To avoid multiple, conflicting results across different measures, a clear primary outcome should be declared, with a corresponding definition. This should be registered on an approved trial registry to maximize transparency and reduce reporting bias. In the meantime, researchers should consider using the composite outcome measure GI‐2 (time to tolerance of oral diet and passage of stool) as a validated, evidence‐based measure that reflects the return of both upper and lower gastrointestinal function. Researchers should also consider the routine collection of quality of life data.
Previous studies have focused on broad definitions of ileus in the setting of colorectal surgery; this may not reveal the full extent of the issue. Return of gastrointestinal function is a challenge in many fields of abdominal (and non‐abdominal) surgery. As the focus of this review is on clinical manifestations (rather than pathophysiological mechanisms), cautious extrapolation of these results across these other surgical areas might still be reasonable. This review was unable to distinguish between measures of ileus and postoperative nausea and vomiting. While these may well represent different aetiological entities, their clinical manifestations share similarities and a pragmatic (patient‐focused) perspective of gastrointestinal function is encouraged. As this review focused on RCTs of gastrointestinal surgery, and excluded studies with an observational design, the benefits of prospective design have to be balanced against the extent of generalizability of the results to an unselected population.
The reporting of postoperative bowel function is limited by unacceptable variation in outcome measures and a paucity of evidence to describe their validity. This undoubtedly contributes to the challenges encountered in identifying and evaluating the return of bowel function after gastrointestinal surgery. An agreed core outcome set is required.
Supporting information
Table S1 Search strategy for published RCTs
Table S2 Primary Registries: clinical trial registries endorsed by the WHO International Clinical Trials Registry Platform (ICTRP) (n = 16)
Table S3 Included manuscripts
Acknowledgements
The authors are grateful to all attendees of the Gastrointestinal Recovery Research Day (28 April 2017 at the Royal College of Surgeons of England, London, UK), supported by the Association of Coloproctology of Great Britain and Ireland Delphi research programme and the Bowel Disease Research Foundation.
Disclosure: The authors declare no conflict of interest.
Funding information No funding
References
- 1. Scarborough JE, Schumacher J, Kent KC, Heise CP, Greenberg CC. Associations of specific postoperative complications with outcomes after elective colon resection: a procedure‐targeted approach toward surgical quality improvement. JAMA Surg 2017; 152: e164681. [DOI] [PubMed] [Google Scholar]
- 2. Hughes M, Coolsen MM, Aahlin EK, Harrison EM, McNally SJ, Dejong CH et al Attitudes of patients and care providers to enhanced recovery after surgery programs after major abdominal surgery. J Surg Res 2015; 193: 102–110. [DOI] [PubMed] [Google Scholar]
- 3. Tiernan J, Cook A, Geh I, George B, Magill L, Northover J et al Use of a modified Delphi approach to develop research priorities for the Association of Coloproctology of Great Britain and Ireland. Colorectal Dis 2014; 16: 965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapman SJ, Pericleous A, Downey C, Jayne DG. Postoperative ileus following major colorectal surgery. Br J Surg 2018; 105: 797–810. [DOI] [PubMed] [Google Scholar]
- 5. Chapman SJ, Wells CI. Challenges in ileus research. Colorectal Dis 2018; 20: 639. [DOI] [PubMed] [Google Scholar]
- 6. Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg 2013; 17: 962–972. [DOI] [PubMed] [Google Scholar]
- 7. Gero D, Gié O, Hübner M, Demartines N, Hahnloser D. Postoperative ileus: in search of an international consensus on definition, diagnosis, and treatment. Langenbecks Arch Surg 2017; 402: 149–158. [DOI] [PubMed] [Google Scholar]
- 8. Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST et al The COMET Handbook: version 1.0. Trials 2017; 18(Suppl 3): 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirkham JJ, Gargon E, Clarke M, Williamson PR. Can a core outcome set improve the quality of systematic reviews? – a survey of the Co‐ordinating Editors of Cochrane Review Groups. Trials 2013; 14: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d'Agostino MA et al Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol 2014; 67: 745–753. [DOI] [PubMed] [Google Scholar]
- 12. van ’t Hooft J, Duffy JM, Daly M, Williamson PR, Meher S, Thom E et al; Global Obstetrics Network (GONet). A core outcome set for evaluation of interventions to prevent preterm birth. Obstet Gynecol 2016; 127: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahnan K, Tozer PJ, Adegbola SO, Lee MJ, Heywood N, McNair AGK et al; ENiGMA collaborators . Developing a core outcome set for fistulising perianal Crohn's disease. Gut 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolthuis AM, Bislenghi G, Fieuws S, de Buck van Overstraeten A, Boeckxstaens G, D’Hoore A. Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta‐analysis. Colorectal Dis 2016; 18: O1–O9. [DOI] [PubMed] [Google Scholar]
- 15. Read TE, Brozovich M, Andujar JE, Ricciardi R, Caushaj PF. Bowel sounds are not associated with flatus, bowel movement, or tolerance of oral intake in patients after major abdominal surgery. Dis Colon Rectum 2017; 60: 608–613. [DOI] [PubMed] [Google Scholar]
- 16. Venara A, Slim K, Regimbeau JM, Ortega‐Deballon P, Vielle B, Lermite E et al Proposal of a new classification of postoperative ileus based on its clinical impact‐results of a global survey and preliminary evaluation in colorectal surgery. Int J Colorectal Dis 2017; 32: 797–803. [DOI] [PubMed] [Google Scholar]
- 17. van Bree SH, Bemelman WA, Hollmann MW, Zwinderman AH, Matteoli G, El Temna S et al Identification of clinical outcome measures for recovery of gastrointestinal motility in postoperative ileus. Ann Surg 2014; 259: 708–714. [DOI] [PubMed] [Google Scholar]
- 18. Hedrick TL, McEvoy MD, Mythen MMG, Bergamaschi R, Gupta R, Holubar SD et al; Perioperative Quality Initiative (POQI) 2 Workgroup . American Society for Enhanced Recovery and Perioperative Quality initiative joint consensus statement on postoperative gastrointestinal dysfunction within an enhanced recovery pathway for elective colorectal surgery. Anesth Analg 2018; 126: 1896–1907. [DOI] [PubMed] [Google Scholar]
- 19. Yorkshire Surgical Research Collaborative . Multicentre observational study of gastrointestinal recovery after elective colorectal surgery. Colorectal Dis 2018; 20: 536–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search strategy for published RCTs
Table S2 Primary Registries: clinical trial registries endorsed by the WHO International Clinical Trials Registry Platform (ICTRP) (n = 16)
Table S3 Included manuscripts


