Figure 1.
Comparison of the Domain Architecture of Plant VG K+ Channels and Metazoan Shaker Channels.
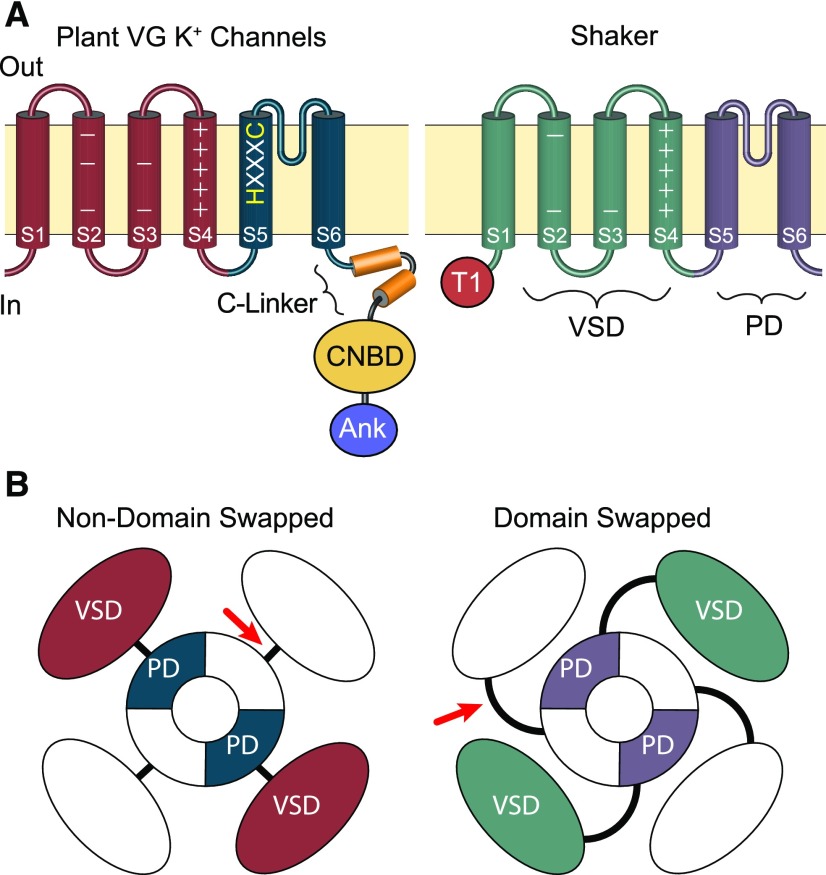
(A) Side view schematic drawings of channel subunits. The plasma membrane is represented by the tan horizontal boxes; extracellular and intracellular sides are marked “Out” and “In”; and transmembrane domains within the subunits are depicted with cylinders. In both channels, the S1-S4 transmembrane segments comprise the voltage sensor domain (VSD, marked on Shaker); the S5-S6 transmembrane segments form the channel PD (marked on Shaker); and the extracellular loop between S5 and S6 forms the pore’s selectivity filter. Basic voltage-sensing gating charges reside in S4 and are indicated by + signs. The S5 of plant voltage-gated K+ channels includes an HXXXC amino acid motif that is highly conserved in CNBD superfamily channels but is absent from Shaker channels. Plant VG K+ channels and Shakers also differ in the composition of their cytoplasmic domains. The C terminus of plant VG K+ channels has a CNBD connected to the PD through a conserved helical linker (C-linker). In contrast, Shaker channels have neither a CNBD nor a C-linker and contain a distinctive N-terminal tetramerization domain (T1), which plays a role in subunit assembly. Many plant VG K+ channels also contain a series of Ankyrin repeats (Ank) in the C terminus distal to the CNBD, which are also absent from Shaker channels.
(B) Aerial view—from the extracellular membrane face—of channel tetramers showing the relative positions of VSDs within the channel tetramers. Structural analysis of multiple metazoan CNBD superfamily channels shows the VSD is positioned directly adjacent to the PD from the same subunit and connected by a short S4-S5 linker (red arrow); this arrangement is therefore likely to be conserved in plant VG K+ channels. For Shaker lineage channels, the VSD is domain-swapped and sits nearest the neighboring subunit’s PD, although it still gates the PD from the same subunit through an extended S4-S5 linker (red arrow).