Abstract
Background:
Food and Drug Administration–approved daptomycin dosing uses actual body weight, despite limited dosing information for obese patients. Studies report alterations in daptomycin pharmacokinetics and creatine phosphokinase elevations associated with higher weight-based doses required for obese patients. Limited information regarding clinical outcomes with alternative daptomycin dosing strategies in obesity exists.
Objective:
This study evaluates equivalency of clinical and safety outcomes in obese patients with daptomycin dosed on adjusted body weight versus a historical cohort using actual body weight.
Methods:
This retrospective, single center study compared equivalency of outcomes with two one-sided tests in patients with body mass index ⩾30 kg/m2 who received daptomycin dosed on actual body weight versus adjusted body weight. The primary outcome was clinical failure. Secondary outcomes included 90-day readmission and 90-day mortality. A combined safety endpoint included creatine phosphokinase elevation, patient-reported myopathy, and rhabdomyolysis.
Results:
A total of 667 patients were screened for inclusion; 101 patients were analyzed with 50 in the actual body weight cohort and 51 in the adjusted body weight cohort. The two regimens were statistically equivalent for clinical failure (2% actual body weight versus 4% adjusted body weight; p < 0.001 for equivalency). The two regimens were also statistically equivalent for 90-day mortality (6% actual body weight versus 4% adjusted body weight; p = 0.0014 for equivalency). Limitations include single center, retrospective design, and sample size. Daptomycin dosing intensified throughout the study period.
Conclusion:
The two daptomycin dosing cohorts were statistically equivalent for both clinical failure and 90-day mortality. More data are needed to assess outcomes with higher (⩾8 mg/kg/day) daptomycin doses in this patient population.
Keywords: creatine phosphokinase, daptomycin, dosing, obesity
Introduction
In 2015, it was estimated that 36.5% of the US population was obese, defined as a body mass index (BMI) of ⩾30 kg/m2.1 Obese individuals not only have an increase in morbidity and mortality from infections but are also at an increased risk for developing nosocomial infections.2,3 In addition, obesity has been identified as an independent risk factor for mortality in patients with critical illness.2 While antibiotic treatment plays a pivotal role in decreasing this risk of mortality, the question has often been raised of optimal antibiotic dosing to ensure positive outcomes.4
Antimicrobial dosing in the setting of obesity remains a challenge as drug distribution alterations can occur, and this patient population is often underrepresented in clinical trials.2 Drug distribution is a complex system affected by body composition, regional blood flow, and protein binding.2 To further complicate this matter, renal drug clearance may be increased in obese populations due to higher glomerular surface area.2 One such antibiotic which is affected by these alterations is the bactericidal lipopeptide, daptomycin.4
Daptomycin is used to treat serious Gram-positive infections caused by organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE).5 In order to exhibit bactericidal activity, a sufficient area under the concentration-time curve (AUC) of daptomycin to mean inhibitory concentration (MIC) ratio is required.5–7 For bactericidal effect, free daptomycin concentrations should average two to four times the MIC over a 24-h period.7 When obtaining Food and Drug Administration (FDA) approval, daptomycin was dosed at 4–6 mg/kg of actual body weight (ABW) based on indication.5,6 However, obese patients with a BMI ⩾30 mg/m2 were not well represented in the clinical trials.5 It is recognized that dosing daptomycin by ABW results in an increased AUC and maximum concentration (Cmax); toxicity has been linked to trough concentration, so provided that drug clearance is appropriate, the consequences of dosing on ABW may be minimal.2,8 Conflicting data are available regarding the risk of adverse events in the setting of obesity.9,10 Figueroa and colleagues9 published a retrospective study which showed at mean daptomycin doses of 8 mg/kg, creatine phosphokinase (CPK) elevations were not statistically higher in the obese population versus normal weight individuals, although this study was limited in size. A more recent publication by Dare and colleagues10 identified both obesity and statin co-administration as independent risk factors for rhabdomyolysis.
Despite this, pharmacokinetic studies indicate that daptomycin AUC is increased in obesity, while drug clearance and volume of distribution are not statistically different when based on ABW.5,11,12 Monte Carlo simulations have confirmed that daptomycin dose is directly related to risk for CPK elevation.12 In addition, daptomycin dosing based on ABW for patients weighing >111 kg has been associated with CPK elevations at 7 days.5 Unpublished abstracts utilizing Monte Carlo simulations report that daptomycin dosing based on an adjusted body weight (AdjBW) using a correction factor of 0.4 and calculated as ideal body weight (IBW) + 0.4 (ABW – IBW) provides an AUC/MIC ratio more closely correlated with normal weight individuals receiving 6 mg/kg dosing for endocarditis treatment.13 Due to these simulation results, daptomycin dosing using AdjBW has been suggested for patients with morbid obesity.13
Clinical outcomes data related to alternative daptomycin dosing strategies in the setting of obesity are limited. Ng and colleagues14 published a single center retrospective study comparing the use of daptomycin dosed with IBW to ABW. No differences in clinical outcomes were detected; however, this study limited doses to the 4–6 mg/kg based on FDA indication.14 A single published abstract supports daptomycin dosing with AdjBW for patients >130% of their IBW.15 No statistical difference in clinical effectiveness was detected comparing patients dosed with AdjBW and a historical control dosed with ABW.15 Evidence regarding optimal dosing strategies for daptomycin to limit adverse effects while optimizing clinical outcomes is lacking, especially considering the recent use of doses ⩾8 mg/kg in clinical practice to target higher MICs or difficult-to-penetrate sites of infection.8,9,12 A recently published guideline for antibiotic dosing in the setting of obesity recommended dosing daptomycin utilizing AdjBW with a correction factor of 0.4, but this recommendation was based primarily on expected pharmacokinetic alterations, with limited clinical outcomes.16
This study aims to compare two different daptomycin dosing strategies in the setting of obesity to help provide further guidance regarding appropriate dosing. The main objective of this study is to compare clinical failure and microbiologic cure between obese patients receiving daptomycin dosed using AdjBW versus a historical cohort dosed using ABW.
Methods
This single center, retrospective trial tested the statistical equivalence of clinical outcomes for obese patients who received daptomycin dosed on AdjBW based on a newly implemented hospital protocol (April 2014–December 2015) versus a historical cohort receiving daptomycin dosed on ABW (December 2012–2013). The study received approval from the institutional review board. Patients were identified by daptomycin order during the pre-specified time frames and data were collected via chart review.
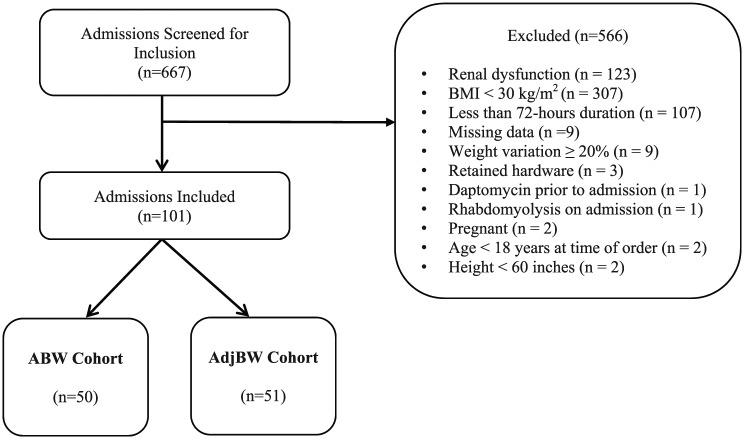
Patients 18 years of age or older with a BMI ⩾30 kg/m2 who received daptomycin for at least 72 h were included in the analysis. Infections with documented retained surgical hardware or lead infections with pacemakers that were not removed were excluded. Patients meeting any of the following renal dysfunction criteria were excluded: creatinine clearance ⩽30 ml/min calculated by Cockcroft Gault using AdjBW calculated by [IBW + 0.4(ABW − IBW)] at any point during the course of therapy, continuous renal replacement therapy (CRRT), hemodialysis (HD), or peritoneal dialysis (PD). Patients with microbiologic isolates that were identified as not susceptible to daptomycin were excluded. Additional exclusion criteria are listed in Figure 1.
Figure 1.
Patients evaluated for inclusion.
ABW: actual body weight; AdjBW: adjusted body weight; BMI: body mass index.
The primary outcome assessed was clinical failure, defined as the development of resistance as noted on subsequent culture results [isolates that were resistant or non-susceptible by the Clinical and Laboratory Standards Institute (CLSI) such as Enterococcus MIC ⩾4 µg/ml or Staphylococcus aureus MIC >1 µg/ml] or recurrent signs or symptoms of infection necessitating antibiotic therapy modification as documented in the patient’s electronic medical record. Secondary outcomes included microbiologic success, defined as at least one documented culture showing microbiologic eradication and no evidence of subsequent clinical failure, readmission at 90 days, and mortality at 90 days. Readmission and mortality data beyond the index admission were only evaluated for patients returning to the study site for treatment with the primary reason for admission being the infection previously treated with daptomycin.
A composite safety endpoint including CPK elevation, patient-reported myopathy, and rhabdomyolysis was compared between groups. CPK elevation was defined as ⩾3 times the upper limit of normal for patients with a normal baseline and ⩾5 times the upper limit of normal for patients without a baseline value as defined previously in available literature.6,14 Patient-reported myopathy was determined by reviewing clinician progress notes. Rhabdomyolysis was defined as an elevation in CPK concentration (as defined above), plus a positive urine myoglobin or acute kidney injury indicated by an increase in SCr by ⩾0.3 mg/dl within 48 h or an increase in serum creatinine (SCr) to ⩾1.5 times baseline, which occurred within the prior 7 days or provider documentation of rhabdomyolysis within the medical record. Information regarding concomitant statin therapy and intensity of statin therapy during the course of daptomycin is reported along with baseline characteristics.
Patient demographic and clinical characteristics including race, age, sex, height, and ABW were recorded, along with daptomycin dose and duration of therapy were collected. Daptomycin dose in mg/kg was provided within the medication order and rounded by pharmacy per institutional policy to the nearest 50 mg. If the calculated dose was between 500 and 550 mg, then 500 mg or 1 vial was dispensed. IBW was calculated using 45.5 + (height in inches >60 × 2.3) for females and 50 + (height in inches >60 × 2.3) for males, AdjBW [IBW + 0.4 (ABW − IBW)], and BMI were calculated for each patient included. The indication for antibiotic therapy, microbiological isolates, and daptomycin MIC were collected, if available in the medical record.
Descriptive and inferential statistics were utilized. Categorical data were reported as number (percent) and analyzed using asymptotic or exact Pearson’s chi-square tests depending on expected cell counts. Shapiro-Wilk tests of normality were performed on continuous variables and are not reported. All continuous variables are reported as median (interquartile range) and were analyzed using Mann–Whitney U tests, a non-parametric test akin to a two independent sample t-test.
Statistical methods used in hypotheses of superiority or differences between groups are not appropriate for equivalency studies.17 To evaluate the equivalency of primary and secondary outcomes between groups, we used two one-sided tests (TOST) for categorical data.17 The percent of each group experiencing the outcome was reported, along with the percent (risk) difference between groups and 90% confidence interval (CI). We established a priori that an acceptable margin between groups was 15%. Equivalency margins for infectious diseases treatments are based upon clinical judgment for an acceptable decrease in effectiveness in exchange for an improvement in safety and tolerability.18 Previous retrospective evaluations have indicated that 70% of equivalency studies within the discipline of infectious diseases utilize a margin of 10–15%.18 Equivalency tests reverse the interpretation of the p value and values <0.05 are deemed statistically equivalent. TOST report p values for both the upper and lower margins. If the p values were different, then the greater of the two was referred to as the overall p value for the tests. All analyses were performed using SAS software, Version 9.4 of the SAS System for Windows (SAS Institute, Cary, NC, USA).
Results
Of the 667 patients screened, 101 met inclusion criteria with 50 patients included in the ABW cohort and 51 patients in the AdjBW cohort (Figure 1).
Patient characteristics are detailed in Table 1. The majority of patients included were White and the median age was 51 years. The most common infections were osteomyelitis, skin and soft tissue infections (SSTI), and abscess. Patients included in the AdjBW cohort were prescribed higher mg/kg doses than in the ABW cohort. The most common microbial isolates were MRSA and VRE. Daptomycin dosing over the time period analyzed was statistically different between the two cohorts with higher doses being prescribed in the AdjBW cohort (p < 0.001; Table 1).
Table 1.
Comparison of ABW versus AdjBW groups.
| Variable | ABW group (n = 50) | AdjBW group (n = 51) | p value for difference |
|---|---|---|---|
| Age (years) | 51 (38–57) 20, 78 |
51 (44–59) 19, 76 |
0.401a |
| Males | 23 (46) | 26 (51) | 0.617b |
| Race | 0.014c | ||
| African American | 8 (16) | 2 (4) | |
| Hispanic | 1 (2) | 2 (4) | |
| Native American | 2 (4) | 0 (0) | |
| White | 34 (68) | 32 (63) | |
| Unknown/missing | 5 (10) | 15 (29) | |
| BMI category | 0.092b | ||
| Class I: (30.0–34.9 kg/m2) | 24 (48) | 14 (27) | |
| Class II: (35.0–39.9 kg/m2) | 11 (22) | 18 (35) | |
| Class III: (⩾40.0 kg/m2) | 15 (30) | 19 (37) | |
| BMI (kg/m2) | 35 (32–43) | 37 (34–43) | 0.187a |
| 30, 69 | 30, 61 | ||
| Length of stay (days) | 21 (10–39) | 25 (14–36) | 0.315a |
| 6, 88 | 3, 123 | ||
| Duration of therapy (days) | 8.0 (5.0–42.0) | 11.5 (5.0–29.0) | 0.563a |
| 3, 56 | 3, 56 | ||
| Indication | 0.593c | ||
| UTI | 5 (10) | 3 (5.8) | |
| SSTI | 8 (16) | 4 (7.8) | |
| Abscess | 6 (12) | 6 (11.8) | |
| Osteomyelitis | 20 (40) | 11 (21.6) | |
| Endocarditis | 0 (0) | 3 (6) | |
| Bacteremia | 3 (6) | 12 (23.5) | |
| Intra-abdominal infection | 4 (8) | 5 (9.8) | |
| Neutropenic fever | 3 (6) | 3 (6) | |
| Empiric | 1 (2) | 4 (7.8) | |
| Dose category prescribed (mg/kg) | ABW, n (%) | AdjBW, n (%) | |
| Low ⩽6 mg/kg/day 5.4 ± 0.8 mg/kg/d (mean ± standard deviation) |
41 (82) | 11 (22) | 0.001b |
| Medium 6.1–8.0 mg/kg/day 7.5 ± 0.6 mg/kg/d (mean ± standard deviation) |
7 (14) | 23 (45) | |
| High >8.0 mg/kg/day 8.9 ± 0.9 mg/kg/d (mean ± standard deviation) |
2 (4) | 17 (33) |
ABW: actual body weight; AdjBW: adjusted body weight; BMI: body mass index; SSTI: skin and soft tissue infection; UTI: urinary tract infection.
Values represent median (interquartile range) and minimum, maximum, or number (%).
Mann–Whitney U test.
Pearson’s chi-square.
Exact Pearson’s chi-square.
The two treatment arms were statistically equivalent for the primary endpoint of clinical failure between the ABW cohort and the AdjBW cohort, p < 0.001 (Table 2). Ninety-day mortality rates observed in the ABW and AdjBW cohorts were statistically equivalent, p < 0.0014. Rates of readmission within 90 days were not statistically equivalent between cohorts and favored the AdjBW cohort (20% ABW cohort versus 10% AdjBW cohort; p = 0.2470) (Table 2). A post hoc power analysis using the results from our primary endpoint of clinical failure was performed using the TOSTER package19 in R version 3.5.0.20 Using an alpha of 0.05, overall sample size of 101, and clinical failure rates of 3.1% and 7.7%, a margin of 15% was powered at 90%.
Table 2.
Comparison of efficacy endpoints.
| Clinical endpoints | ABW, n (%) | AdjBW, n (%) | Risk difference (90% confidence interval) | Two one-sided tests p values | ||
|---|---|---|---|---|---|---|
| Lower margin | Upper margin | Conclusion | ||||
| Clinical failurea | 1 (2.0) | 3 (6.1) | 3.9 (– 2.4 to 10.6) | <0.0001 | <0.0001 | Equivalent |
| Developed resistance | 1 (2.0) | 2 (3.9) | 1.9 (–3.6 to 7.5) | <0.0001 | <0.0001 | Equivalent |
| Antibiotic therapy modification | 0 (0) | 2(4) | 4.0 (0.6 to 8.6) | <0.0001 | <0.0001 | Equivalent |
| 90-day mortality | 3 (6.0) | 2 (3.9) | −2.1 (–9.2 to 5.0) | 0.0014 | <0.0001 | Equivalent |
| 90-day readmission | 10 (20.0) | 5 (9.8) | −10.2 (–21.8 to 1.4) | 0.2470 | 0.0002 | Not equivalent |
ABW: actual body weight; AdjBW: adjusted body weight.
A total of four patients met criteria for clinical failure; one patient met criteria based on both components of the definition; that is, antibiotic therapy was modified due to clinical signs and symptoms and subsequent cultures indicated development of antimicrobial resistance to daptomycin.
Combined developed resistance and antibiotic therapy modification.
Microbiologic data including isolates identified and information available for patients meeting definition of microbial success are presented in Table 3. Of the total number of patients who met criteria for clinical failure; two patients had documented MRSA isolates with initial MICs of 0.5. The remaining two patients had VRE isolates, one with an initial MIC of 2 and the other with an initial MIC of 4.
Table 3.
Microbial isolates and microbial success.
| Organism | ABW, n (%) | AdjBW, n (%) | ||||
|---|---|---|---|---|---|---|
| MRSA | 8 (30.8) | 10 (31.3) | ||||
| MSSA | 4 (15.4) | 0 (0) | ||||
| MRSE | 2 (7.7) | 3 (9.4) | ||||
| VRE | 8 (30.8) | 19 (59.4) | ||||
| Enterococcus sp. | 4 (15.4) | 0 (0) | ||||
| Clinical endpoints | ABW (n = 18) | AdjBW (n = 26) | Risk difference (90% confidence interval) | Two one-sided test p values | ||
| Lower margin | Upper margin | Conclusion | ||||
| Microbiologic successa | 10 (55.6) | 18 (69.2) | 13.7 (–10.7 to 38.0) | 0.0264 | 0.4643 | Not equivalent |
ABW: actual body weight; AdjBW: adjusted body weight; MRSA: methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis; MSSA: methicillin-susceptible Staphylococcus aureus; VRE: vancomycin-resistant enterococci.
Documented microbial eradication (i.e. clean follow-up cultures – bacteremia).
All patients were assessed for the safety of daptomycin therapy. Results for CPK elevation, myopathy, and rhabdomyolysis, as well as a combined safety endpoint are presented in Table 4.
Table 4.
Comparison of safety endpoints.
| Variable | ABW, n (%) | AdjBW, n (%) | Risk difference (90% confidence interval) | Two one-sided test p values | ||
|---|---|---|---|---|---|---|
| Lower margin | Upper margin | Conclusion | ||||
| CPK elevation | 2 (8.0) | 7 (17.5) | 9.5 (–0.1 to 22.8) | 0.0012 | 0.2484 | Not equivalent |
| Myopathy | 1 (2.0) | 3 (5.9) | 3.9 (–2.4 to 10.2) | <0.0001 | 0.0019 | Equivalent |
| Rhabdomyolysis | 3 (6.0) | 2 (3.9) | −2.1 (–9.2 to 5.0) | 0.0014 | <0.0001 | Equivalent |
| Combined safety endpoint | 5 (10.0) | 9 (17.7) | 7.7 (–3.6 to 18.9) | 0.0004 | 0.1404 | Not equivalent |
ABW: actual body weight; AdjBW: adjusted body weight; CPK: creatine phosphokinase.
Overall, the two regimens were not statistically equivalent for the combined safety endpoint when comparing the ABW and AdjBW cohorts (10% versus 18%, respectively) but were statistically equivalent in the individual components of patient-reported myopathy or incidence of rhabdomyolysis (Table 2). The incidence of CPK elevations was higher in the AdjBW population and did not meet statistical equivalence (8% ABW cohort versus 18% AdjBW cohort).
Discussion
To our knowledge, this is the first published clinical investigation comparing daptomycin doses based on AdjBW to ABW in obese patients. Our study showed that clinical failure and 90-day mortality were statistically equivalent when comparing ABW to AdjBW dosing strategies for daptomycin. Ninety-day readmission and microbiologic success were not statistically equivalent between the two regimens and favored the AdjBW cohort. The combined safety endpoint including: CPK elevation, patient-reported myopathy, and rhabdomyolysis was not statistically equivalent between the two groups. Overall, there is paucity of data related to clinical outcomes using either daptomycin dosed by an IBW or AdjBW in the setting of obesity, particularly considering the higher total doses now used in clinical practice.14,15 Ng and colleagues14 compared clinical outcomes utilizing daptomycin dosed at 4–6 mg/kg IBW versus ABW in obese patients and found no differences in microbiologic cure, length of stay, or safety. Their study institution’s surveillance of staphylococcus and enterococci cultures and sensitivities revealed that an appropriate AUC/MIC ratio would be achieved >98% of the time with dosing based on IBW prior to protocol implementation.14 The dosing protocol using IBW was developed in an attempt to limit toxicities with a known highly susceptible bacterial ecology.14 Our institution implemented the AdjBW protocol for daptomycin in obesity based on available pharmacokinetic data, in an attempt to limit toxicities, and potentially reduce costs. No surveillance of cultures was performed. At the time of implementation, there was no published data on clinical endpoints utilizing AdjBW for dosing daptomycin in obese patients.
In 2017, two studies were published highlighting the importance of utilizing high doses of daptomycin dosed by ABW in the setting of VRE bacteremia.21,22 Britt and colleagues21 were able to demonstrate a mortality benefit when a high (>10 mg/kg) dose of daptomycin was used compared to lower doses. In addition, Chuang and colleagues22 were able to demonstrate a 40% mortality reduction with each mg/kg increase in daptomycin dose. While both of these studies were conducted in a non-obese population, they highlight the importance of daptomycin dose in treating serious infections with VRE.21,22 In 2018, a study was published showing the impact and importance of daptomycin dose on overall survival in a Veterans Affairs population with MRSA bacteremia.23 Clinical outcomes were compared in patients who received ⩾7 mg/kg ABW daptomycin dose and those who received 6 mg/kg ABW daptomycin dose for MRSA bacteremia.23 The study revealed that propensity scored-matched 30-day morality significantly favored the patients who received ⩾7 mg/kg ABW dose.23 The overall population was not obese in this study; however, in the propensity score-matched cohort, the ⩾7 mg/kg groups’ median BMI exceeded 30 kg/m2.23 In 2015, the Infectious Disease Society of America (IDSA) and American Heart Association (AHA) recommended daptomycin 10–12 mg/kg for the treatment of VRE endocarditis.24 IDSA has also recommended daptomycin doses of 8–10 mg/kg for the treatment of serious MRSA infections including osteomyelitis, endocarditis, and bacteremia.25 Available literature for clinical endpoints using IBW for daptomycin dosing is only available for doses of 4–6 mg/kg/day, which highlights the necessity of further study as higher doses (⩾8 mg/kg/day) are prescribed in clinical practice.14
Our institution chose to dose daptomycin based on AdjBW, as there was concern regarding the clinical implications of under-dosing antibiotic therapy. Given that daptomycin received initial approval utilizing ABW, the appropriateness of dosing an obese individual by IBW while continuing to utilize ABW for non-obese individuals was questioned. With the use of much higher doses than originally approved, additional research evaluating safe and effective daptomycin dosing in the setting of obesity is warranted.
Inherent limitations to this study include a single study site and retrospective design. A relatively small sample of patients was included; however, the size is comparable to other published studies evaluating safety or efficacy of daptomycin.8,14 Patients were not matched; however, there were no statistical differences noted when baseline characteristics were compared. Indications for antimicrobial therapy were analyzed initially as a group and then assessed individually in a stepwise approach to determine whether the increased number of patients with osteomyelitis in the ABW population and increased number of patients with bacteremia in AdjBW population met statistical significance; neither comparison met statistical significance. We acknowledge that this difference could still be clinically significant, particularly considering the increase in numbers for patients with osteomyelitis, who are at risk for readmission. This numerical difference could impact the finding for readmission at 90 days. We also recognize that while it would be ideal to study one indication or one microbe, we chose to include multiple sites of infection to avoid limiting sample size and mimic a real-world population. Including multiple sites of infection is also congruent with previous published literature assessing IBW dosing for daptomycin.14
We also acknowledge that changes in practice at the study site may have influenced results, including the adoption of clinical monitoring software in 2014 which prompted clinicians to order CPK concentrations at baseline and every 7 days while receiving daptomycin therapy. The increase in CPK elevations noted in the AdjBW cohort could be reflective of increased clinical monitoring. While the incidence of the combined safety endpoint was significantly higher in the AdjBW group, we believe this finding was driven by the increase in CPK elevations secondary to an increase in clinical monitoring.
It is also noted that while daptomycin mg/kg dosing increased over the time frame analyzed for many indications, absolute doses in both cohorts remained within FDA-approved dosing of 4–6 mg/kg ABW. The overall mean dose of daptomycin based on ABW for each individual was 5.6 mg/kg in the ABW versus 5.5 mg/kg in the AdjBW cohort. This indicates that despite contemporary clinical practice recommending higher mg/kg doses for certain infections, using an adjustment factor in the setting of obesity can result in a total dose received consistent with FDA-approved dosing based on ABW. The similar absolute mg/kg dose received (based on ABW) could explain our findings which indicated that both dosing approaches were statistically equivalent for clinical failure and 90-day mortality. Significantly more patients were prescribed ⩾8 mg/kg in the AdjBW dosing arm potentially due to differences in clinical practice and clinicians reacting to the institutional protocol change and concern for under-dosing with the AdjBW approach. When considering our findings combined with recent data highlighting mortality benefits with higher doses in both VRE bacteremia and MRSA bacteremia, our impression is that a comparison of clinical outcomes stratified by indication and daptomycin dose in obese patients dosed with an AdjBW and non-obese patients should be conducted.21–23 As literature has been published favoring higher doses of daptomycin with improved mortality, this is a vital clinical question to ensure that obese populations receiving alternative daptomycin dosing strategies are not experiencing suboptimal clinical outcomes.21–23
Despite these limitations, AdjBW remains a reasonable option for dosing obese patients with similar infection distribution given our findings of statistical equivalence for clinical failure and combined safety endpoints. Pharmacokinetic data, such as higher AUC and Cmax and unchanged Vd in obesity, support using an AdjBW to limit toxicity and achieve similar drug concentrations as in a non-obese population.8,11,12 This study has the potential to influence daptomycin prescribing practices and identifies a gap in the literature regarding daptomycin dosing based on AdjBW in obese patients.
Conclusion
Although small in number, clinical failure rates were statistically equivalent between the two dosing cohorts. The combined safety endpoint was also statistically equivalent when comparing the two dosing strategies. Based on our clinical outcomes, coupled with published pharmacokinetic data, dosing daptomycin using AdjBW appears to be a reasonable alternative. More data are needed to determine outcomes of dosing daptomycin using AdjBW in an obese population receiving recently recommended higher doses (⩾8 mg/kg/day). A comparison of obese patients receiving daptomycin dosed with AdjBW to non-obese controls stratified by indication and dose is warranted.
Acknowledgments
The authors thank Rachel Musgrove, Pharm.D., and Jamie L. Miller, Pharm.D., who provided feedback on manuscript clarity. The results of this study were presented in its entirety as an abstract and poster at the 2017 American College of Clinical Pharmacy (ACCP) Meeting in Phoenix, AZ, USA.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
ORCID iD: Ashley N. Fox  https://orcid.org/0000-0002-6269-797X
https://orcid.org/0000-0002-6269-797X
Contributor Information
Ashley N. Fox, Department of Pharmacy: Clinical and Administrative Sciences, College of Pharmacy, The University of Oklahoma, Oklahoma City, OK, USA.
Winter J. Smith, Department of Clinical Sciences, The Ben and Maytee Fisch College of Pharmacy, The University of Texas at Tyler, Tyler, TX, USA
Katherine E. Kupiec, Department of Clinical Pharmacy, OU Medical Center, Oklahoma City, OK, USA
Stephanie J. Harding, Department of Clinical Pharmacy, Wesley Medical Center, Wichita, KS, USA
Beth H. Resman-Targoff, Department of Pharmacy: Clinical and Administrative Sciences, College of Pharmacy, The University of Oklahoma, Oklahoma City, OK, USA
Stephen B. Neely, Office of Instructional Science and Assessment, College of Pharmacy, The University of Oklahoma, Oklahoma City, OK, USA
Bryan P. White, Department of Clinical Pharmacy, OU Medical Center, Oklahoma City, OK, USA
Ryan E. Owens, Department of Pharmacy Practice, Wingate University School of Pharmacy, Hendersonville, NC, USA
References
- 1. Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015; 219: 1–8. [PubMed] [Google Scholar]
- 2. Pai MP, Bearden DT. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 2007; 27: 1081–1091. [DOI] [PubMed] [Google Scholar]
- 3. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis 2006; 6: 438–446. [DOI] [PubMed] [Google Scholar]
- 4. Alobaid AS, Hites M, Lipman J, et al. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: a structured review. Int J Antimicrob Agents 2016; 47: 259–268. [DOI] [PubMed] [Google Scholar]
- 5. Bookstaver PB, Bland CM, Qureshi ZP, et al. Safety and effectiveness of daptomycin across a hospitalized obese population: results of a multicenter investigation in the southeastern United States. Pharmacotherapy 2013; 33: 1322–1330. [DOI] [PubMed] [Google Scholar]
- 6. Bhavnani SM, Rubino CM, Ambrose PG, et al. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: data from a randomized trial of patients with bacteremia and endocarditis. Clin Infect Dis 2010; 50: 1568–1574. [DOI] [PubMed] [Google Scholar]
- 7. Safdar N, Andes D, Craig WA. In vivo pharmacodynamic activity of daptomycin. Antimicrob Agents Chemother 2004; 48: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dvorchik BH, Damphousse D. The pharmacokinetics of daptomycin in moderately obese, morbidly obese, and matched nonobese subjects. J Clin Pharmacol 2005; 45: 48–56. [DOI] [PubMed] [Google Scholar]
- 9. Figueroa DA, Mangini E, Amodio-Groton M, et al. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin Infect Dis 2009; 49: 177–180. [DOI] [PubMed] [Google Scholar]
- 10. Dare RK, Tewell C, Harris B, et al. Effect of statin coadministration on the risk of daptomycin-associated myopathy. Clin Infect Dis 2018; 67: 1356–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benvenuto M, Benzinger DP, Yankelev S, et al. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in health volunteers. Antimicrob Agents Chemother 2006; 50: 3245–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhavnani SM, Ambrose PG, Hammel JP, et al. Evaluation of daptomycin exposure and efficacy and safety endpoints to support risk-versus-benefit considerations. Antimicrob Agents Chemother 2015; 60: 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farkas A, Sussman R. Dosing of daptomycin in the morbidly obese: which body weight is it? In: Abstracts of the ID week conference, San Diego, CA, 2012 October, Abstract #1637. [Google Scholar]
- 14. Ng JK, Schulz LT, Rose WE, et al. Daptomycin dosing based on ideal body weight versus actual body weight: comparison of clinical outcomes. Antimicrob Agents Chemother 2014; 58: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shemanski S, Bennett N, Boyd S, et al. 698: evaluation of clinical effectiveness utilizing adjusted body weight for daptomycin dosing. Crit Care Med 2016; 44: 251. [Google Scholar]
- 16. Meng L, Mui E, Holubar MK, et al. Comprehensive guidance for antibiotic dosing in obese adults. Pharmacotherapy 2017; 37: 1415–1431. [DOI] [PubMed] [Google Scholar]
- 17. Castelloe J, Watts D; SAS Institute Inc. Equivalence and noniferiority testing using SAS/STAT® software. Paper SAS1911-2015, SAS: The Power to Know®, http://support.sas.com/resources/papers/proceedings15/SAS1911-2015.pdf (accessed 25 May 2018).
- 18. Li Y, He Y, Sheng Y, et al. Systematic evaluation on non-inferiority and equivalence randomized trials of anti-infective drugs. Expert Rev Anti Infect Ther 2014; 11: 1377–1389. [DOI] [PubMed] [Google Scholar]
- 19. Lakens D. Equivalence tests: a practical primer for t tests, correlations, and meta-analyses. Soc Psychol Personal Sci 2017; 8: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. R and Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 21. Britt NS, Potter EM, Patel N, et al. Comparative effectiveness and safety of standard-, medium-, and high-dose daptomycin strategies for the treatment of vancomycin-resistant Enterococcal bacteremia among Veterans Affairs patients. Clin Infect Dis 2017; 64: 605–613. [DOI] [PubMed] [Google Scholar]
- 22. Chuang YC, Lin HY, Chen PY, et al. Effect of daptomycin dose on the outcome of vancomycin-resistant, daptomycin-susceptible Enterococcus faecium bacteremia. Clin Infect Dis 2017; 64: 1026–1034. [DOI] [PubMed] [Google Scholar]
- 23. Timbrook TT, Caffrey AR, Luther MK, et al. Association of higher daptomycin dose (7 mg/kg or greater) with improved survival with methicillin resistant Staphylococcus aureus bacteremia. Pharmacotherapy 2018; 38: 189–196. [DOI] [PubMed] [Google Scholar]
- 24. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132: 1435–1486. [DOI] [PubMed] [Google Scholar]
- 25. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52: 285–292. [DOI] [PubMed] [Google Scholar]