Abstract
OBJECTIVE
Cisplatin (CDDP) is an anti-neoplastic agent that has been used in treatments of both pediatric and adult cancers. It has many side effects, such as ototoxicity, nephrotoxicity, and neurotoxicity. Lipoplatin (LIPO) is a nanomolecule with 110 nm diameter and composed of lipids and CDDP. In this study, we aimed to compare the toxic effects of LIPO with CDDP in the cochlear cells with anti-tumoral doses determined in neuroblastoma cells.
MATERIALS and METHODS
House Ear Institute Organ Corti 1 (HEI-OC1), MYC-N amplified KELLY, and MYC-N non-amplified SH-SY5Y human neuroblastoma cells were used in this study. Firstly, anti-tumoral lethal dose 50 (LD50) of LIPO and CDDP were determined using the WST-1 assay in both neuroblastoma cells. Then anti-tumoral doses of CDDP and LIPO were applied on HEI-OC1 cells for evaluating the toxic effects. The apoptotic cell death was measured using flow cytometric analysis of annexin-V/7-amino-actinomycin (7-AAD) and cell cycle tests.
RESULTS
LIPO or CDDP inhibited cell viability in a dose- and time-dependent manner in both neuroblastoma and HEI-OC1 cells. LD50 values were selected as 20 mM for CDDP and 750 mM for LIPO in neuroblastoma cells. After the 48-hour incubation, KELLY cells treated with 20 mM CDDP and 750 mM LIPO had a 53% viability; SH-SY5Y cells treated 20 mM CDDP and 750 mM LIPO had a 45% and 58% viability, respectively; and HEI-OC1 cells treated with 20 mM CDDP and 750 mM LIPO had a 65% and 82% viability, respectively.
CONCLUSION
LIPO showed less toxic effects in the HEI-OC1 cells compared to CDDP at anti-tumoral doses.
Keywords: Neuroblastoma, Hei-Oc1, lipoplatin, cisplatin, ototoxicity
INTRODUCTION
Neuroblastoma is a common malignancy in childhood and originates from the primitive neural crest cells in the sympathetic nervous system [1–3]. The behavior of neuroblastoma varies according to the presence of the tumor and the status of metastasis [3, 4]. Neuroblastoma constitutes about 8–10% of all childhood cancers and is responsible for 15% of childhood cancer-related deaths [5]. In neuroblastoma, the patient’s age, tumor histology and stage, and cytogenetic and molecular genetic markers are the most important prognostic indicators [3].
Cisplatin (CDDP) is an anti-neoplastic agent that has been used in the treatment of both pediatric and adult cancers [3, 6]. Although CDDP is used in cancer treatments, it has many side effects, such as ototoxicity, nephrotoxicity, and neurotoxicity [6, 7]. Although nephrotoxicity can be controlled and diminished after CDDP treatment, there is no prevention modality against CDDP ototoxicity in clinical practice [8, 9]. CDDP causes damage to the organ of corti in the cochlea, resulting hearing loss, which can lead to social development deficiencies, such as learning problems, in children. Therefore, ototoxicity is an extremely serious problem in childhood [3, 7]. In previous studies, we reported that CDDP shows toxic effects on House Ear Institute-Organ Corti 1 (HEI-OC1) cells [7, 10].
Liposomal platinum is the most promising drug formulation under clinical conditions [11]. Lipoplatin (LIPO) is a liposomally encapsulated form of CDDP [12]. LIPO is a nanomolecule of 110 nm in diameter, which is composed of lipids and CDDP [13]. LIPO has been shown to have toxicity lower than CDDP and more drug accumulation in the tumor [12, 14].
In the present study, we aimed to compare the anti-tumor response of LIPO and CDDP in neuroblastoma and the toxic effects in HEI-OC1 cells.
MATERIALS and METHODS
This study was in vitro study. This study was performed in accordance with the principles of the Declaration of Helsinki. Informed consent is not required for the current in vitro study.
Cell Culture and Reagents
HEI-OC1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% (w/v) L-glutamine. The cells were incubated at 33°C in a 10% CO2 incubator [6]. KELLY (worse prognostic, MYC-N amplified) and SH-SY5Y (good prognostic, non-MYC-N amplified) neuroblastoma cells (DSMZ) were maintained in DMEM (Gibco™) and Rosewell Park Memorial Institute (RPMI)-1640 (Gibco™) containing 10% FBS, 1% penicillin/streptomycin, and 1% L-glutamine. The cells were incubated at 37° C and 5% CO2 conditions [5, 15, 16]. All reagents were freshly prepared with mediums before all experiments. The cell viability was determined using an automatic cell counter with a trypan blue exclusion test.
Detection of Cell Proliferation
The WST-1 assay used to determine cell proliferation is based on the basic principle the conversion of the pink tetrazolium salt (WST-1) to a dark red formazan dye via the succinate-tetrazolium reductase enzyme, which is active only in living cells in the mitochondrial respiratory chain [7]. Mitochondrial dehydrogenase enzyme activity is increased as the number of viable cells increases. As enzyme activity increases, formazan dye production also increases. Therefore, the formazan dye increases in direct proportion to the number of metabolically active cells in a cell culture. Cells were counted and 104 cells/well were seeded with least 6 replicates in a 96-well plate by overnight incubation. The cells were then treated with different concentrations of LIPO and CDDP (10 μM-1000 μM) for 24, 48, and 72 hours. Following the incubation periods, the WST-1 assay was performed by adding 10 μL of WST reagents (Roche) to each well, and HEI-OC1 and KELLY and SH-SY5Y neuroblastoma cells were further incubated at 33°C and 37°C, respectively, for 2 hours. After incubation periods, the absorbances of the cells was measured test wavelength at 450 nm and the reference wavelength at 630 nm was measured at 630 nm on an ELISA reader (Thermo Scientific). The half-maximal inhibitory or lethal concentrations (IC50 or LD50) of the agents were calculated according to control cells viability.
Detection of Apoptotic Cells
Apoptotic cell death was determined using annexin-V-fluorescein isothiocyanate (FITC)/7-amino-actinomycin D (7-AAD; BD Biosciences) and Cycletest™ Plus DNA Reagent Kit (BD Biosciences) in all cells. In the normal cells, phosphatidylserine molecules on the cytoplasmic surface of the cell membrane migrate to the outer surface of the cell membrane in the event of cell apoptosis. Annexin-V can be labeled with FITC, a fluorescent substance, which can bind to the phosphatidylserine on the outer surface of the cell [17]. Thus, the apoptotic cells to which FITC is conjugated with annexin-V can be made visible. The principle of the method is based on of the extent apoptosis determination using flow cytometry of cells stained with annexin-V-FITC and 7-AAD, a non-vital dye.
The auditory cells and tumor cells were incubated for 48 hours in the presence of LIPO or CDDP. In KELLY neuroblastoma cells, LIPO was used at 750 μM and CDDP was used at 20 μM concentrations determined from cell proliferation assays. In SH-SY5Y cells, LIPO was used at 750 μM and CDDP used at 20 μM. In the auditory cells, cells were treated with 750 μm LIPO and 20 μM CDDP. After incubation, cells were collected in a 1.5 mL centrifuge tube and centrifuged at 500 × g for 10 minutes. Each cell pellet was re-suspended in 100 μL phosphate-buffered saline (PBS), and subsequently 5 μL of annexin-V and 5 μL 7-AAD were added on the cells. The cells were incubated in the dark at 4°C for 15 minutes according to protocol. After incubation, 400 μL annexin-V binding buffer was added in each tube. Analyses were carried out using a flow cytometer (Accuri, BD) at 488 nm excitation and 530 nm emission wavelengths for annexin-V and 488 nm excitation and 647 nm emission wavelengths for 7-AAD, and evaluated using the BD Accuri C6 Software (BD Biosciences) [6].
Cells at different stages of the cell cycle were determined using Cycletest™ Plus DNA Reagent Kit (BD). After the agent applications, the cells were washed thrice with PBS and re-suspended in the medium, incubated for 15 minutes at room temperature with propidium Iodide, and then analyzed using flow cytometry [18, 19].
Statistical Analysis
The Statistical Package for Social Sciences software for Windows, version 15.0, released 2007 (SPSS Inc.; Chicago IL, USA) was used for statistical evaluation of the data. Findings with a p value <0.05 for statistical significance were accepted. Non-parametric Mann-Whitney U test was also used. Each experiment was repeated at least in triplicate for statistical evaluation.
RESULTS
Cell Proliferation Results
The cell viability of KELLY human MYC-N amplified and SH-SY5Y human MYC-N non-amplified neuroblastoma cells treated with different doses of LIPO (10–1000 μM) and CDDP (10–1000 μM) was determined using the WST-1 assay. LIPO and CDDP inhibited neuroblastoma cell proliferations in a dose- and time-dependent manner (Figure 1, 2).
Figure 1.
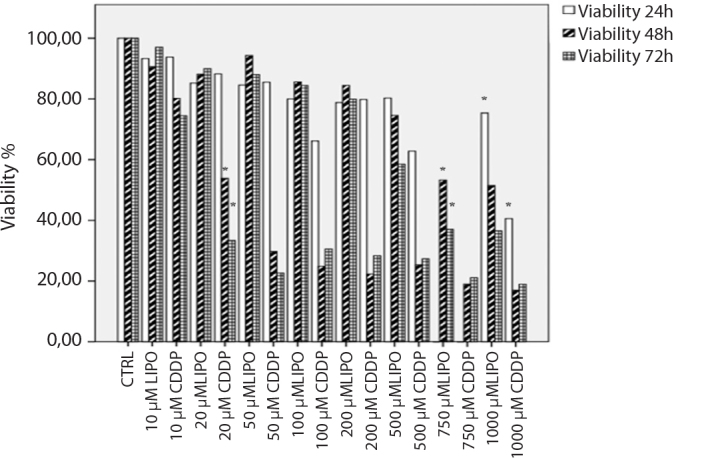
Relative cell proliferation assay results of CDDP and LIPO in KELLY (MYC-N amplified) human neuroblastoma cells for 24, 48, and 72 hour incubations. CDDP and LIPO significantly decreased the cell viability with 1000 μM concentrations after 24 hours of incubation compared to control cells (p<0.05). CDDP and LIPO significantly decreased the cell viability with 20 and 750 μM concentrations after 48 hours of incubation compared to control cells (p<0.05). CDDP and LIPO significantly decreased the cell viability with 20 and 750 μM concentrations after 72 hours of incubation compared to control cells (p<0.05).
CDDP: cisplatin; LIPO: lipoplatin
Figure 2.
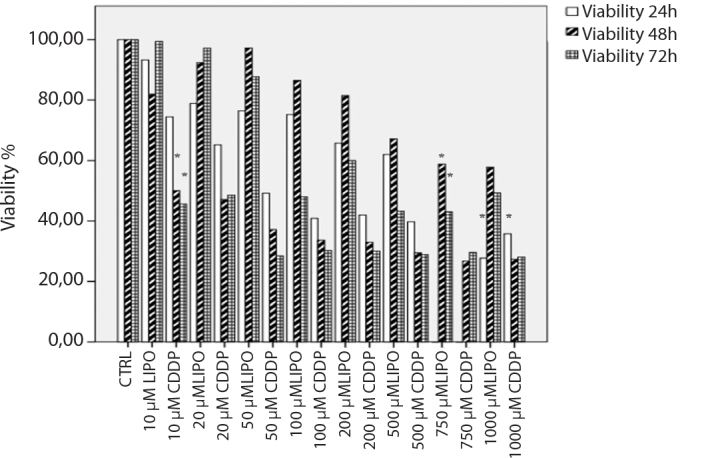
Relative cell proliferation assay results of CDDP and LIPO in SH-SY5Y (MYC-N non-amplified) human neuroblastoma cells for 24-, 48-, and 72-hour incubations. CDDP and LIPO significantly decreased the cell viability with 1000 μM concentrations after 24 hours of incubation compared to control cells (p<0.05). CDDP and LIPO significantly decreased the cell viability with 10 and 750 μM concentrations after 48 hours of incubation compared to control cells (p<0.05). CDDP and LIPO significantly decreased the cell viability with 20 and 750 μM concentrations after 72 hours of incubation compared to control cells (p<0.05).
CDDP: cisplatin; LIPO: lipoplatin
The doses of CDDP and LIPO applied for KELLY and SHSY cells were also applied for HEI-OC1 cell and cell viability was determined by the WST-1 assay. Also, proliferations of HEI-OC-1 cells were decreased with lower doses of CDDP but a higher dose of LIPO treatments.
In KELLY MYC-N amplified human neuroblastoma cells, the cell viability decreased at a rate of 41% and 75% with the treatment of 1000 μM CDDP and LIPO, respectively, after 24 hours of incubation(p<0.05; Figure 1).
Cisplatin inhibited 50% cell viability at 20 μM doses, while 750 μM LIPO inhibited 50% cell viability in KELLY cells after 48 hours of incubation (p<0.05; Figure 1).
Also, 20 μM CDDP decreased KELLY cell viability at 33%, but 750 μM LIPO inhibited cell viability at 37% after 72 hours of incubation (p<0.05; Figure 1).
In the SH-SY5Y MYC-N non-amplified human neuroblastoma cells, cell viability decreased at a rate of 28% and 36% with the treatment of 1000 μM CDDP and LIPO, respectively, after 24 hours of incubation (p<0.05; Figure 2).
Cisplatin inhibited 50% cell viability at 10 μM doses, while LIPO inhibited cell viability at 750 μM in SH-SY5Y cells after 48 hours incubation (p<0.05; Figure 2).
Also, 10 μM CDDP decreased SH-SY5Y cell viability at 45%, but 750 μM LIPO inhibited cell viability at 42% after 72 hours of incubation (p<0.05; Figure 2).
In the HEI-OC1 cells, cell viability decreased at 43% and 87% with the treatment of 1000 μM CDDP and LIPO, respectively, after 24 hours of incubation (p<0.05; Figure 3).
Figure 3.
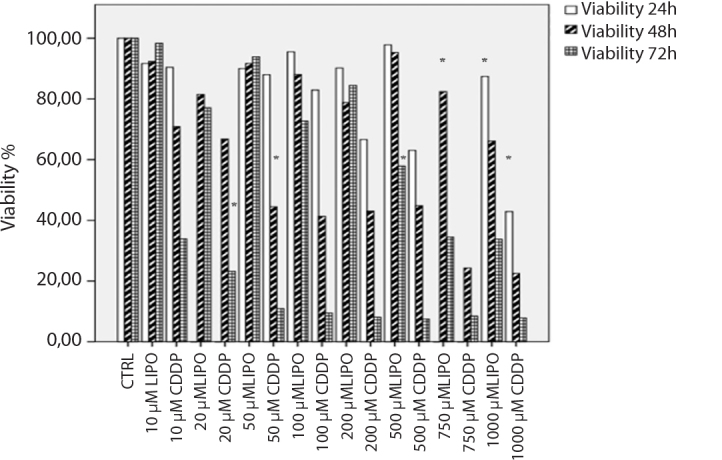
Relative cell proliferation assay results of CDDP and LIPO in HEI-OC1 auditory cells for 24, 48, and 72 hour incubations. CDDP and LIPO significantly decreased the cell viability with 1000 μM concentrations after 24 hours of incubation compared to control cells (p<0.05). CDDP and LIPO significantly decreased the cell viability with 50 and 750 μM concentrations after 48 hours of incubation compared to control cells (p<0.05). CDDP and LIPO significantly decreased the cell viability with 20 and 750 μM concentrations after 72 hours of incubation compared to control cells (p<0.05) (b).
CDDP: cisplatin; LIPO: lipoplatin
Cisplatin inhibited 50% cell viability at 50 μM doses, while LIPO inhibited cell viability at 750 μM in SH-SY5Y cells after 48 hours of incubation (p<0.05; Figure 3).
Also, 20 μM CDDP decreased SH-SY5Y cell viability at 23%, but 500 μM LIPO inhibited cell viability at 58% after 72 hours of incubation (p<0.05; Figure 3).
In this study, the in vitro cytotoxic effects of LIPO and CDDP on HEI-OC1 cells with neuroblastoma cells were also compared. Firstly, HEI-OC1 cells were treated with the same doses of both CDDP and LIPO and the IC50 doses were determined from these experiments.
Moreover, we tested the apoptotic effects of the agents in these cells using the annexin-V and cell cycle analysis.
HEI-OC1 cells treated with 750 μM LIPO for 48 hours had an 82% viability. HEI-OC1 cells treated with 20 μM CDDP for 48 hours had a 65% viability.
Therefore, 20 μM CDDP and 750 μM LIPO concentration applied for 48 hours was designated as the IC50. Treatment with LIPO or CDDP induced a dose-dependent (CDDP, IC50=20 and 50 μM; LIPO IC50=750 and 1000 μM) and time-dependent (48 h) inhibition of cell proliferation.
In the SH-SY5Y cells, the cell viability was 57% for LD50 doses of LIPO 750 μM, the lowest dose of CDDP is 50% of deaths from 10 μM achieved, and the dose increases with decreased cell viability. The cell viability was 53% for LD50 doses of LIPO 750 μM and 53% for the 20 μM CDDP in KELLY cells. In the HEI-OC1 cells, 1000 μM LIPO led to 66% cell viability and 50 μM of CDDP decreased the cell viability below 50% (44%).
Apoptosis Results of the Cells
We also compared apoptosis level at the lC50 concentration of LIPO and CDDP on KELLY, SH-SY5Y, and HEI-OC1 cells. To compare the apoptosis level of LIPO and CDDP, flow cytometry annexin-V/7-AAD and cell cycle assays were used.
LD50 doses of CDDP caused 62.3–77.3% and LIPO caused 38.85–45.6% apoptotic cell death in KELLY cells. In SH-SY5Y, LD50 doses of CDDP caused 75.8–86.9% and LIPO induced 25.3–56.3% apoptosis (Figure 4). Cell cycle findings showed similar results in apoptosis (data not shown).
Figure 4.
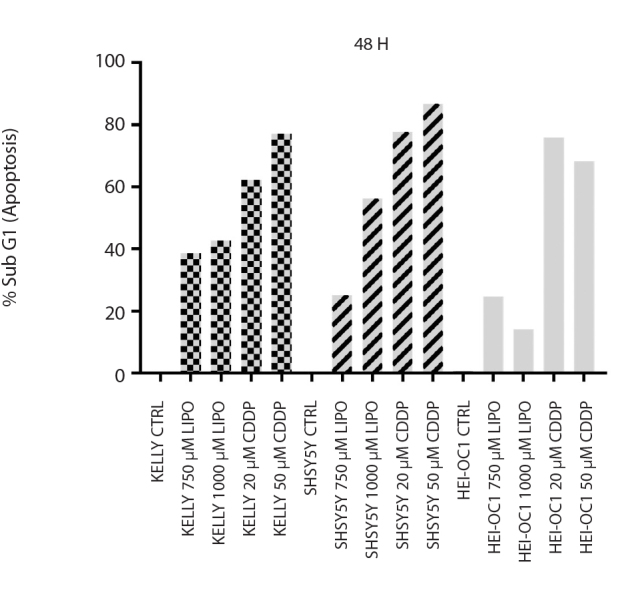
Apoptotic percentages of LIPO (750–1000 μM) and CDDP (25–50 μM) treated HEI-OC1, KELLY, and SH-SY5Y cells determined using flow cytometry analysis
CDDP: cisplatin; LIPO: lipoplatin
DISCUSSION
An ideal chemotherapeutic agent should be effective on cancer cells, and it should not affect normal cells. CDDP is a highly effective chemotherapeutic agent widely used for treating various adult and pediatric cancers; however, it has dose-limiting serious adverse effects, including nephrotoxicity, neurotoxicity, and ototoxicity [1]. It has been shown that CDDP has ototoxic effects in many studies [3,4]. LIPO, a nanomolecule 110 nM diameter, is a liposomal formulation of CDDP and is composed of lipids and CDDP. LIPO was developed to reduce the systemic toxicity of CDDP [12, 13].
Our previous study has shown that CDDP has toxic effects in HEI-OC1 cells [20]. In the present study, we aimed to compare the potential toxic effects of LIPO and CDDP on HEI-OC1 cells. Previous in vitro and in vivo researches have shown that LIPO has less nephrotoxic effects compared to CDDP [12]. Furthermore, chemoradiotherapy with LIPO for advanced gastric cancer treatment demonstrated minor toxicity at phase 1/2 study [12]. Fantini et al. [13] showed that LIPO therapy has lesser renal toxicity than CDDP in lung and breast cancer treatment in clinical studies.
Moreover, LIPO showed anti-tumor effect in CDDP-sensitive- and -resistant ovarian cancer cells since apoptosis was induced by caspases 3, 8, and 9 activation; Bax upregulation; and Bcl-2 downregulation [11]. LIPO caused a synergistic effect combination with doxorubicin and the albumin-bound paclitaxel abraxane. Also, LIPO inhibited ovarian xenograft tumor growth with minimum systemic toxicity in that study.
In an animal study to evaluate the renal toxicities of LIPO and CDDP, it has been shown that LIPO induces less structural and functional damage to the kidneys in mice than CDDP [21]. It was observed that intraperitoneal bolus injection of CDDP and LIPO in rat kidneys resulted in less platinum accumulation with LIPO, although CDDP and LIPO reached the same level of platinum.
CONCLUSION
In this study, the anti-tumor and apoptotic effect of LIPO was determined in neuroblastoma cells at a higher dose than CDDP and at later time periods. LIPO caused less apoptotic cell death on cochlear cells than CDDP at anti-tumoral doses, suggesting a lower toxicity in cochlear cells compared to CDDP. Further in-vivo comparative studies are needed for understanding the mechanism of ototoxic effects of LIPO versus CDDP [22].
Acknowledgements
The authors would like to thank Prof. F. Kalinec for ensuring HEI-OC1 cells.
Footnotes
This study was presented at the 48th Congress of the International Pediatric Oncology (SIOP 2016 Congress), 19–22 October, Dublin, Ireland.
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013)..
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Concept - N.O., S.A., Z.A., E.S.; Design - E.S., Z.A.; Supervision - N.O., S.A., Z.A.; Resource - N.O., Z.A.; Materials - Z.A, S.A., E.S.; Data Collection and/or Processing - E.S.; Analysis and/or Interpretation - E.S.; Literature Search - E.S.; Writing - E.S., Z.A.; Critical Reviews - Z.A., S.A., N.O., E.Ç.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Louis CU, Shohet JM. Neuroblastoma: molecular pathogenesis and therapy. Annu Rev Med. 2015;66:49–63. doi: 10.1146/annurev-med-011514-023121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–79. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 3.Olgun N, Kansoy S, Aksoylar S, Cetingul N, Vergin C, Oniz H, et al. Experience of the Izmir Pediatric Oncology Group on Neuroblastoma: IPOG-NBL-92 Protocol. Pediatr Hematol Oncol. 2003;20:211–8. doi: 10.1080/713842276. [DOI] [PubMed] [Google Scholar]
- 4.Castleberry RP. Biology and treatment of neuroblastoma. Pediatr Clin North Am. 1997;44:919–37. doi: 10.1016/S0031-3955(05)70537-X. [DOI] [PubMed] [Google Scholar]
- 5.Dodurga Y, Gundogdu G, Tekin V, Koc T, Satiroglu-Tufan NL, Bagci G, et al. Valproic acid inhibits the proliferation of SHSY5Y neuroblastoma cancer cells by downregulating URG4/URGCP and CCND1 gene expression. Mol Biol Rep. 2014;41:4595–9. doi: 10.1007/s11033-014-3330-3. [DOI] [PubMed] [Google Scholar]
- 6.Cecen E, Ercetin P, Kirkim G, Pamukoglu A, Aktas S, Altun Z, et al. Apoptotic Effects of Sanguinarine on the Organ of Corti 1 Cells: Comparison with Cisplatin. J Int Adv Otol. 2015;11:19–22. doi: 10.5152/iao.2015.484. [DOI] [PubMed] [Google Scholar]
- 7.Altun Z, Olgun Y, Ercetin P, Aktas S, Kirkim G, Serbetcioglu B, et al. Protective effect of acetyl-l-carnitine against cisplatin ototoxicity: role of apoptosis-related genes and pro-inflammatory cytokines. Cell Prolif. 2014;47:72–80. doi: 10.1111/cpr.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altun ZS, Gunes D, Aktas S, Erbayraktar Z, Olgun N. Protective effects of acetyl-L-carnitine on cisplatin cytotoxicity and oxidative stress in neuroblastoma. Neurochem Res. 2010;35:437–43. doi: 10.1007/s11064-009-0076-8. [DOI] [PubMed] [Google Scholar]
- 9.Gunes D, Kirkim G, Kolatan E, Guneri EA, Ozogul C, Altun Z, et al. Evaluation of the effect of acetyl L-carnitine on experimental cisplatin ototoxicity and neurotoxicity. Chemotherapy. 2011;57:186–94. doi: 10.1159/000323621. [DOI] [PubMed] [Google Scholar]
- 10.Olgun Y, Kirkim G, Altun Z, Aktas S, Kolatan E, Kiray M, et al. ProtectiveEffect of Korean Red Ginseng on Cisplatin Ototoxicity: Is It EffectiveEnough? J Int Adv Otol. 2016;12:177–83. doi: 10.5152/iao.2016.1989. [DOI] [PubMed] [Google Scholar]
- 11.Casagrande N, Celegato M, Borghese C, Mongiat M, Colombatti A, Aldinucci D. Preclinical activity of the liposomal cisplatin lipoplatin in ovarian cancer. Clin Cancer Res. 2014;20:5496–506. doi: 10.1158/1078-0432.CCR-14-0713. [DOI] [PubMed] [Google Scholar]
- 12.Tippayamontri T, Kotb R, Paquette B, Sanche L. Efficacy of cisplatin andLipoplatin in combined treatment with radiation of a colorectal tumor in nude mouse. Anticancer Res. 2013;33:3005–14. [PubMed] [Google Scholar]
- 13.Fantini M, Gianni L, Santelmo C, Drudi F, Castellani C, Affatato A, et al. Lipoplatin treatment in lung and breast cancer. Chemother Res Pract. 2011;2011:125192. doi: 10.1155/2011/125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makar AB, McMartin KE, Palese M, Tephly TR. Formate assay in body flu ids: application in methanol poisoning. Biochem Med. 1975;13:117–26. doi: 10.1016/0006-2944(75)90147-7. [DOI] [PubMed] [Google Scholar]
- 15.Cecen E, Altun Z, Ercetin P, Aktas S, Olgun N. Promoting effects of sanguinarine on apoptotic gene expression in human neuroblastoma cells. Asian Pac J Cancer Prev. 2014;15:9445–51. doi: 10.7314/APJCP.2014.15.21.9445. [DOI] [PubMed] [Google Scholar]
- 16.Petroni D, Tsai J, Mondal D, George W. Attenuation of low dose methylmercury and glutamate induced-cytotoxicity and tau phosphorylation by an N-methyl-D-aspartate antagonist in human neuroblastoma(SHSY5Y) cells. Environ Toxicol. 2013;28:700–6. doi: 10.1002/tox.20765. [DOI] [PubMed] [Google Scholar]
- 17.Edebali N, Tekin IO, Acikgoz B, Acikgoz S, Barut F, Sevinc N, et al. Apoptosis and necrosis in the circumventricular organs after experimentalsubarachnoid hemorrhage as detected with annexin V and caspase 3immunostaining. Neurol Res. 2014;36:1114–20. doi: 10.1179/1743132814Y.0000000437. [DOI] [PubMed] [Google Scholar]
- 18.Schmid I, Krall WJ, Uittenbogaart CH, Braun J, Giorgi JV. Dead cell discriminationwith 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992;13:204–8. doi: 10.1002/cyto.990130216. [DOI] [PubMed] [Google Scholar]
- 19.Pietkiewicz S, Schmidt JH, Lavrik IN. Quantification of apoptosis andnecroptosis at the single cell level by a combination of Imaging Flow Cytometry with classical Annexin V/propidium iodide staining. J ImmunolMethods. 2015;423:99–103. doi: 10.1016/j.jim.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Altun Z, Pamukoğlu A, Olgun Y, Aktaş S, Çetinayak HO, Kırkım G, et al. Acetyl-L-Carnitine protects HEI-OC1 auditory cells from radiation andcisplatin induced toxicity. Int J Clin Exp Med. 2016;9:13605–14. [Google Scholar]
- 21.Devarajan P, Tarabishi R, Mishra J, Ma Q, Kourvetaris A, Vougiouka M, et al. Low renal toxicity of lipoplatin compared to cisplatin in animals. Anticancer Res. 2004;24:2193–200. [PubMed] [Google Scholar]
- 22.Serinan E, Altun Z, Aktas S, Olgun N. Comparison of Cisplatin with Lipoplatin in Terms of Ototoxicity. Pediatr Blood Cancer. 2016;63:S201. doi: 10.5152/iao.2018.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]


