Abstract
Hemodialysis vascular access dysfunction is a common and intractable problem in clinical practice with no definitive therapy yet available. As a key mediator of vascular and cardiac maladaptive remodeling, mineralocorticoid receptor (MR) plays a pivotal role in vascular fibrosis and intimal hyperplasia (IH) and is potentiated locally in hemodialysis vascular access following diverse injuries, like barotrauma, cannulation and shear stress. MR-related genomic and non-genomic pathways are responsible for triggering vascular smooth muscle cell activation, proliferation, migration and extracellular matrix overproduction. In endothelial cells, MR signaling diminishes nitric oxide production and its bioavailability, but amplifies reactive oxygen species, leading to an inflammatory state. Moreover, MR favors macrophage polarization towards a pro-inflammatory phenotype. In clinical settings like post-angioplasty or stenting restenosis, the beneficial effect of MR antagonists on vascular fibrosis and IH has been validated. In aggregate, therapeutic targeting of MR may provide a new avenue to prevent hemodialysis vascular access dysfunction.
Keywords: Mineralocorticoid receptor, Aldosterone, Arteriovenous fistula failure, Intimal hyperplasia, Hemodialysis vascular access dysfunction
Abbreviations: AVF, arteriovenous fistula; AVG, arteriovenous grafts; Ang II, Angiotensin II; AT1R, Angiotensin II type 1 receptor; CKD, chronic kidney disease; ESRD, end-stage renal disease; ECM, extracellular matrix; eNOS, endothelial nitric oxide synthase; EPCs, endothelial progenitor cells; IH, intimal hyperplasia; IL, interleukin; MR, mineralocorticoid receptor; MCP-1, monocyte chemotactic protein-1; MMPs, matrix metalloproteinases; NO, nitric oxide; α-SMA, α-smooth muscle actin; SGK1, serum-and-glucocorticoid regulated kinase1; VSMC, vascular smooth muscle cells; VCAM-1, vascular cell adhesion molecule-1; WSS, wall shear stress
Highlights
-
•
MR signaling is instrumental in both insufficient outward remodeling and exuberant inward remodeling of AVF.
-
•
The effects of MR in VSMC, endothelial cell, and macrophage act synergistically to promote IH and vascular fibrosis in AVF.
-
•
Pharmacological targeting of MR represents a novel therapeutic strategy to prevent hemodialysis vascular access dysfunction.
1. Introduction
Hemodialysis is an effective modality of renal replacement therapy for end-stage renal disease (ESRD). A functional hemodialysis vascular access is critical for successful dialysis procedures. The National Kidney Foundation Kidney Disease Outcome Quality Initiative (KDOQI) guidelines for vascular access [1] recommends the arteriovenous fistula (AVF) as the first choice of vascular access because of its lower rates of infection, fewer complications, and prolonged survival compared to arteriovenous grafts (AVG) and tunneled catheters. However, vascular access is not without problems, as only a minority (26%) of created fistulas were reported to be mature at 6 months and 21% were abandoned without being able to be used [2]. Moreover, the patency rate of primary unassisted fistulas at 6 months was only 64% [2]. The major cause of hemodialysis vascular access dysfunction is vascular stenosis usually at the site of the venous anastomosis. There is evidence suggesting that AVF non-maturation is prone to occur in the setting of pathological changes characterized by vascular fibrosis and intimal hyperplasia (IH). In recent years, substantial progress has been made in understanding the molecular mechanisms underlying IH and vascular fibrosis, which may involve inflammation, uremia, hypoxia, shear stress, and a hypercoagulable state [3] related to disturbed blood flow and injury to the integrity of vessel wall endothelium. Vascular access dysfunction is associated with over activation of α-smooth muscle actin (α-SMA) positive cells, like myofibroblasts and vascular smooth muscle cells (VSMC). Both cell types proliferate and migrate from adventitia or media to intima under the local influence of diverse cytokines, culminating in excess extracellular matrix (ECM) deposition, IH, vascular fibrosis and AVF failure [4]. However, to date, there are still no effective interventional measures to prevent vascular fibrosis or IH and improve the patency of vascular access.
Mineralocorticoid receptor (MR) is a nuclear receptor and transcription factor that is prominently expressed in renal distal tubules and thereby has been traditionally regarded as a key regulator of electrolyte and water homeostasis. MR is also expressed in vascular endothelial cells and VSMC [5], suggesting a role for MR in vascular pathobiology separate from controlling water-electrolyte balance. Indeed, there is ample evidence that MR contributes to vascular inflammation, fibrosis, and calcification [6,7], as well as VSMC proliferation, migration [8] and the subsequent narrowing of the vascular lumen. MR in endothelial cells, VSMC, and macrophages has been associated with cardiovascular disease and in conjunction promotes vascular inflammation, VSMC activation, and ECM accumulation. MR is upregulated in vein grafts [9] and in dysfunctional AVF [10]. Furthermore, MR blockade is associated with reduction of intima-media thickness, inflammatory infiltration and fibrosis [11] and genetic knockout of MR is able to attenuate IH and vascular fibrosis. Therefore, it is conceivable to speculate that MR also plays a crucial role in hemodialysis vascular access dysfunction, though appropriate precautions should be taken to extrapolate preclinical findings to AVF in humans. Here, we review recent literature related to the potential role of MR in hemodialysis vascular access dysfunction with a focus on AVF stenosis.
2. Vascular access maturation and failure
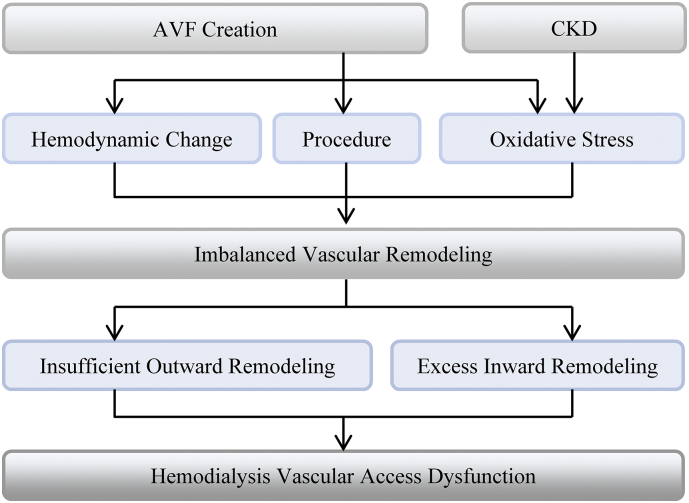
Following AVF surgery, it takes approximately 6 weeks for the fistula to achieve clinical and ultrasonographic maturation [12]. As to gross morphology, a matured AVF is characterized by increased blood vessel diameter and wall thickness especially within the venous segment of the fistula. The process of AVF maturation is complicated, consisting of both outward and inward remodeling. Insufficient outward remodeling and exuberant inward remodeling will lead to a lack of dilatation and neointima thickness (Fig. 1). Driving forces for these changes may include the drastic hemodynamic change to the venous segment of the fistula (barotraumatic injury), inflammation, oxidative stress, and surgical injury [13,14]. In addition, uremic toxins are also associated with vascular injury and AVF non-maturation. In support of this, more than 70% of pre-access veins in ESRD patients have significant hyperplasia at the time of surgery [15]. However, recent studies demonstrated that the contribution of pre-existing IH to AVF nonmaturation is likely very limited, as shown by Martinez et al. [4] and also by the Hemodialysis Fistula Maturation (HFM) Group [16], which is the largest multicenter prospective study of AVF with standardized training for data collection and ultrasound measurements and uniform criteria for defining stenosis. As for post-operative hyperplasia, its role in AVF non-maturation and failure has been less studied. However, more and more evidence suggests that the effects of IH on AVF non-maturation seem to have been overestimated [4,17,18]. To date, it remains uncertain and controversial if post-operative IH is a causative factor for AVF failure and if post-operative intimal hyperplasia is a primary cause or simply a worsening factor for AVF failure. To prove these, multicenter, large scale and adequately-powered clinical studies are definitely needed in the future. Nevertheless, these types of studies will be likely very difficult, if not impossible, because a postoperative sample can only be obtained in those fistulas that require a surgical intervention, limiting our ability to systemically compare with AVFs that mature successfully. On histology, IH predominately consists of myofibroblasts, contractile smooth muscle cells, macrophages [19], and excess ECM molecules like collagen and fibronectin [20]. Vascular fibrosis is driven by improper excess ECM deposition and accumulation [4]. Vascular remodeling occurs when tunica adventitia, media, or intima is injured, and starts immediately after AVF creation when a vein is abruptly subjected to an arterial hemodynamics, or following endothelial denudation due to hemodynamic changes or oxidative stress secondary to uremia in ESRD patients. However, in response to sustained hemodynamic changes, mechanical injury, vascular inflammation, or uremia-induced oxidative stress, aberrant or maladaptive vascular remodeling will take place and result in AVF dysfunction.
Fig. 1.
Schematic diagram of pathological process leading to hemodialysis vascular access dysfunction. Abbreviations:AVF, arteriovenous fistula; CKD, chronic kidney disease.
2.1. Inward remodeling in AVF
Hemodynamic changes represent a crucial factor in vascular remodeling. When a vein is exposed to an arterial environment after arteriovenous anastomosis, blood flow increases, accompanied with two kinds of wall shear stress (WSS), i.e. unidirectional laminal WSS and oscillatory WSS. Oscillatory WSS is more prone to occur in the anastomotic region where AVF stenosis preferentially forms in forearm fistulas. Endothelial cells are essential for WSS-induced vascular remodeling. Unidirectional sheer stress stimulates endothelial nitric oxide synthase (eNOS) response, followed by increasing nitric oxide (NO) release [21], which improves vascular dilatation and attenuates cell proliferation and migration, thereby facilitating adaptive wall remodeling and vasodilatation. In contrast, the oscillatory WSS activates pathways that mitigate NO generation and matrix metalloproteinases (MMPs) release, resulting in insufficient vasodilatation, excessive neointimal hyperplasia, and even vascular stenosis. Patients with chronic kidney disease (CKD) are susceptible to IH possibly because they have a lower NO production and NO bioavailability due to increased oxidative stress. In agreement, the incidence of renal insufficiency was almost three times greater in patients with failed or failing saphenous vein grafts than in patients with patent saphenous vein grafts, implying CKD as a risk factor for maladaptive remodeling of vein grafts [22]. In the setting of AVF, endothelial cells are activated and express a multitude of inflammatory mediators, such as interleukin (IL)-6, IL-8, and monocyte chemotactic protein-1 (MCP-1), which attract and facilitate inflammatory infiltration to the fistula. In turn, inflammatory cells and macrophages/monocytes recruited to AVF or vein grafts produce diverse inflammatory mediators [23], thus establishing a vicious cycle. In addition, increased expression of osteopontin, a matricellular protein present in ECM [20], and CD44, a cellular adhesion molecule [24], during AVF maturation may facilitate macrophages infiltration. Infiltrating macrophages play a vital role in neointimal formation by reinforcing VSMC migration and proliferation through releasing cytokines like tumor necrosis factor α (TNF-α), IL-1, and IL- 1β [25]. Moreover, local inflammatory infiltration can be potentiated by cytokines like macrophage migration inhibitory factor, which is associated with IH and vascular dysfunction in patients with ESRD [26] and polytetrafluoroethylene grafts [27].
2.2. Outward remodeling in AVF
To achieve adequate blood flow, AVF maturation needs an adaptive change in vascular luminal caliber. IH or thrombus narrows vascular lumen. However, the final luminal caliber is determined not only by the degree of hyperplasia or thrombus, but also by the extent of vascular outward expansion that depends on media and adventia elasticity [14,28]. Venous diameter in AVF increases two or three fold during AVF maturation due to outward remodeling. As an immediate sensor of hemodynamic changes, endothelia may play a key role in vascular outward remodeling [15]. In support of this, AVF outward remodeling has been associated with the vasodilatory factor NO, which is released by vascular endothelium in response to hemodynamic stress. NO relaxes VSMC and activates MMPs, which in combination with degradation of internal elastic lamina are important for outward remodeling [29,30]. Indeed, the brachial flow-mediated dilation, which depends on the ability of the endothelium to release the endogenous vasodilator NO, is significantly associated with 6-week postoperative AVF flow rate and diameter [31]. However, these results do not imply that the same happens after anastomosis in the venous limb of the fistula. Further, the brachial flow-mediated dilation assesses NO-mediated dilation in the artery, not in veins. More studies are required to assess the exact role of NO in AVF remodeling and dysfunction. In addition, post-operative medial fibrosis is also likely a risk factor for AVF failure, as evidenced by a number of new studies [4,32]. However, all these findings were derived from a single center, underpowered small study and only limited to the two-stage AVFs that were all done by a single vascular surgeon, thus limiting the generalizability. Despite these limitations, these findings clearly indicate that AVF non-maturation is more likely to occur in the settings of both post-operative venous intimal hyperplasia and medial fibrosis.
3. MR
As a steroid receptor, MR is a member of the nuclear receptor superfamily. In the absence of cognate ligand, MR is primarily distributed in the cytoplasm and bound to chaperone proteins or actin [33]. Once activated by mineralocorticoid hormones like aldosterone, MR is liberated and translocated to nuclei, where it subsequently regulates the expression of numerous target genes. MR is constitutive in certain cells like in the cortical collecting duct of the kidney but experiments from our laboratory have clearly shown that MR can be induced after an injury both in cells where it already exists and in cells where it normally is absent or expressed in trace amounts [10,34,35].
The common view is that only mineralocorticoids like aldosterone and deoxycorticosterone activate MR but we now know that under certain circumstances, glucocorticoids like cortisol and corticosterone can also activate MR. Moreover, recent studies have suggested that Angiotensin II (Ang II) via Ang II type 1 receptor (AT1R) [6], and Rho family small GTPase Rac1 may also be able to transactivate MR in a ligand-independent way [36]. In addition to the well-recognized expression in distal renal tubules, MR is also abundantly expressed in both epithelial tissues and non-epithelial tissues. By immunohistochemistry, Lombes et al. demonstrated that MR is expressed in endothelial cells and VSMC in large blood vessels [37]. The MR expressed in VSMC and endothelial cells is functional and able to regulate gene expression, suggesting that vasculature is also aldosterone-responsive.
3.1. MR activation in vascular remodeling
The pathobiologic activity of aldosterone has been confirmed in multiple extra-renal organ systems, such as immune cells and vasculature, in concert with the verified expression of MR in macrophages, endothelial cells and VSMC. MR expressed in VSMC or endothelial cells can be activated by physiologic circulating concentrations of aldosterone, present in most ESRD patients with AVF [11]. Aldosterone may also contribute to perivascular inflammation, oxidative stress, followed by vascular hyperplasia and fibrosis in an MR-mediated manner [38]. Conversely, MR antagonists confer a beneficial effect on improving diverse cardiovascular diseases and on preventing vascular and cardiac remodeling, as shown by a number of clinical studies [39,40]. Additionally, there is emerging evidence in support of the beneficial effect of MR blockades on IH and vascular fibrosis. For instance, eplerenone, a highly selective MR antagonist, ameliorates constrictive remodeling and collagen accumulation in coronary arteries after angioplasty or stent implantation in animal models [41,42]. Likewise, the classical MR antagonist spironolactone [11] and nonsteroidal MR antagonist finerenone [43] were recently shown to be capable of attenuating IH [44] and vascular fibrosis [45] both in vivo and vitro. Apart from its role in artery remodeling, MR also plays a key role in vein remodeling, as evidenced by a number of studies. For instance, Bafford et al. found that MR is expressed in human venous smooth muscle cells and plays an important role in vein graft arterialization [9]. This was further supported by preclinical evidence that MR inhibition with spironolactone reduced vein graft thickening and inflammation and improved vein graft remodeling in vivo [11], underscoring the potential to use MR antagonists as novel treatments to preserve vein graft patency. In addition, the crosstalk between the aldosterone-MR pathway and Ang II-AT1R pathway may also play an important role in IH and fibrosis. Aldosterone is able to upregulate the activity of AT1R [46]. In turn, AT1R is also essentially involved in aldosterone-MR signaling [47]. Two single nucleotide polymorphisms of AT1R, rs275653 and rs1492099, responsible for increased expression of AT1R, are associated with increased risk of AVF dysfunction [48]. Clinical data suggest that patients with AVG or AVF who have been treated with angiotensin-converting enzyme inhibitor or angiotensin receptor blockade [49] are more likely to have lower complication rates and longer duration of event-free patency [50].
4. Direct role of MR in AVF or AVG dysfunction
4.1. Role of VSMC-specific MR in vascular access dysfunction
Procedures like AVF surgery, which often involve mechanical vessel stretch and manipulation, as well as vascular access cannulation, which inevitably incurs vascular injury, may affect the long-term outcomes of hemodialysis vascular access [14,51]. Lee et al. compared vein tissues collected at the time of new access surgery with those from stenotic AVF/AVG and found that different mechanistic pathways responding to surgical injury were associated with distinct cellular changes within the neointima [52]. However, the biological significance of this study is uncertain because the correlation between the thickness of the pre-existing and post-operative hyperplasia was not demonstrated later in a large cohort [15]. The cellular and molecular changes of veins in response to vascular injury, which could mediate IH, have also been validated. Some CKD patients may already have pre-existing IH and vascular inflammation. Wasse et al. provided further supportive evidence when they collected vein specimens from stage 4 and 5 CKD patients at the time of AVF surgery. Inflammatory mediators like IL6, transforming growth factor-β, TNF-α, and markers of DNA oxidative damage were already present [53]. Vascular wall injury is associated not only with primary hemodialysis vascular access stenosis, but also with reappearance of vascular access stenosis after endovascular interventions [54]. The injury-induced IH and vascular fibrosis may be dependent on MR in VSMC. Aldosterone enhances vascular remodeling following mechanical injury and MR blockade attenuates VSMC activation and reduces neointima formation both in vitro and in vivo [11,43]. In terms of the direct role of VSMC-specific MR in vascular remodeling in response to injury stimulation, Pruthi et al. found that aldosterone-induced VSMC proliferation in murine models of wire-induced carotid injury could be diminished by 79% after the inducible VSMC-specific deletion of MR, highlighting the important role of VSMC specific MR signaling for aldosterone-induced vascular remodeling after vascular injury [55]. Ang II-elicited vascular oxidative stress was also mitigated in mice with VSMC-specific deletion of MR, an additional benefit [56].
The cellular signaling pathway of aldosterone-activated VSMC in vascular remodeling is the subject of major interest. MR activation is able to trigger both non-genomic actions, including increased phosphorylation of mitogen-activated protein kinase, GSK3β and c-Src, and slow genomic actions, such as up-regulation of placental growth factor signaling and oxidative stress signaling pathways via increased transcription of vascular endothelial growth factor type 1 receptor and endothelin. These pathways [57] are integrated to activate VSMC and promote its migration, proliferation and ECM production, leading to vascular intima-media thickness, narrowing of vascular lumen and vascular fibrosis.
Recently, micro-RNAs have been identified to act via epigenetic regulation. As a key mediator of MR-regulated vascular remodeling, miR-29b in VSMC may have a protective effect on vascular remodeling by suppressing the expression of ECM components like collagen and by inhibiting VSMC migration. The abundance and activities of miR-29b in VSMC are diminished when MR is activated [58]. The novel insight into micro-RNA regulation of relevant signaling pathways in vascular remodeling underscores a brand new molecular mechanism underlying MR-mediated IH and fibrosis.
4.2. Role of macrophage-specific MR in vascular access dysfunction
Uncommitted macrophages (M0) can be polarized into two distinct phenotypes: the classical (M1) and the alternative (M2) macrophages, depending on the local cytokine milieu. M1 exhibits a pro-inflammatory activity by releasing pro-inflammatory cytokines, such as MCP-1, TNF-α, IL-6, etc. In contrast, M2 demonstrates an anti-inflammatory activity and promotes tissue repair [59]. In experimental AVF outflow vein specimens from mice with CKD, M1 macrophages dominate. After clodronate-mediated macrophages depletion, M1 infiltration in AVF was abolished and M2 was inversely increased, associated with amelioration of neointima formation as compared with vehicle-treated animals [3]. There is evidence suggesting that M2 macrophages seem to play a key role during adaptive venous remodeling in the early phase of AVF maturation by counteracting aberrant IH [24,60]. Activation of MR in macrophages by aldosterone favors M1 polarization. MR knockout can abolish this effect, denoting a modulatory effect of MR on macrophage phenotypes [61]. In addition, macrophages/monocytes recruited to AVF or AVG may further amplify inflammation by releasing pro-inflammatory cytokines, which promote VSMC proliferation, migration and ECM overproduction, and exacerbate IH and vascular fibrosis. This process is also likely regulated by MR. In support of this concept, myeloid-specific MR knockout in mice drastically suppressed the expression of pro-inflammatory cytokine (IL-6, IL-8 and IL-1β), osteopontin, receptors of MCP1, chemokine (C-C motif) receptor 2, and chemokine (C-C motif) receptor 4 in injured vessels. A lower recruitment of macrophages/monocytes, mitigated VSMC activation, improved neointimal hyperplasia and decreased fibrotic response are all associated with this suppressed response [61,62]. How MR regulates macrophages polarization and expression of pro-inflammatory cytokines remains obscure, but there is evidence suggesting the involvement of the serum-and-glucocorticoid-regulated kinase1 (SGK1) signaling, which has been traditionally regarded to mediate the slow genomic mineralocorticoid actions that regulate electrolyte homeostasis [63]. SGK1 signaling in the vein graft after mechanical injury appears responsible for neointima formation [64] and to contribute to macrophages differentiation towards the pro-inflammatory phenotype [62,65]. The activity of SGK1 signaling pathway is blocked in macrophages derived from myeloid-specific MR knockout mice, marked by a reduced phosphorylation of SGK1. Moreover, quintessential pro-inflammatory signaling pathways, such as activator protein-1 signaling and nuclear factor-κB signaling are all suppressed in macrophages derived from myeloid-specific MR knockout mice. These effects can be overridden in macrophages with SGK1 reconstitution, again accentuating the essential role of SGK1 in MR-mediated regulation of macrophage phenotypes in IH and vascular fibrosis [66].
4.3. Role of endothelial MR in vascular access dysfunction
In the setting of ESRD, oxidative stress is systemically elevated and this may promote VSMC activation and the subsequent MR dependent AVF stenosis [67]. In addition, excess reactive oxygen species in the circulation or locally in AVF may scavenge NO, which is a crucial vascular dilatory factor generated by eNOS when coupling with tetrahydrobiopterin, a critical cofactor for NO production [68]. NO mediates vasodilatation and inhibits VSMC activation when diffusing into media, and suppresses inflammation and thrombosis by acting locally on vascular endothelial cells. MR antagonists have been shown to restore NO production and bioavailability by attenuating eNOS uncoupling in humans and animals [69,70], potentially associated with increased levels of tetrahydrobiopterin. Conversely, endothelial MR activation by aldosterone is able to suppress glucose-6-phosphate dehydrogenase expression, resulting in excessive production of reactive oxygen species [71]. In addition, studies have shown that the aldosterone-MR signaling pathway in endothelial cells promotes production of inflammatory cytokines and superoxide, leading to vascular inflammatory response and oxidative stress [72]. By using DNA microarray analysis, Sekizawa et al. discovered that aldosterone at physiological levels significantly up-regulates the expression of 12 genes in human endothelial cells, which have pleiotropic effects and can be categorized into three subgroups that are respectively related to inflammation, angiogenesis and remodeling based on their biophysiological function. The activating effects on these aldosterone-sensitive genes could be totally prevented by spironolactone [73].
An intact and continuous endothelium serves as a line of defense against thrombosis. Damaged endothelium will trigger the coagulation cascade. Endothelial MR has been shown to be associated with thrombosis. To this end, exogenous aldosterone treatment was found to promote thrombus formation after vascular injury in an MR dependent manner [74]. However, the action of endothelial MR on thrombosis is likely context-dependent: It seems that the pro-thrombotic effect of aldosterone only occurs upon endothelial denudation; In contrast, when endothelium is intact, MR overexpression in endothelial cells turns out to be antithrombotic and this is seemingly associated with up-regulation of endothelial cell protein C receptor and the consequent protein C activity [75].
MR activation in human coronary artery endothelial cells promotes expression of inflammatory mediators like vascular cell adhesion molecule-1 (VCAM-1) [76], which promote endotheliitis by boosting leukocyte entrapment. Patients with thrombosed AVF demonstrate increased expression of VCAM-1 within the fistula [77]. The level of soluble VCAM-1, a fragment of VCAM-1, also appears elevated. Studies confirmed that soluble VCAM-1 could serve as a predictive marker for increased risk of AVF thrombosis in children undergoing hemodialysis [78].
5. Therapeutic strategies and Clinical Implications
Hemodialysis vascular access dysfunction continues to be a paramount challenge for clinical practice with no definitive treatment available yet. A number of approaches have been applied to address this issue, including improving surgical skills, enhancing postoperative care, and adaptive forearm/hand exercise as well as pharmaceutical interventions.Far infrared therapy has been shown to improve patency of AVF, but the effectiveness needs to be validated by large scale trials [79]. Although statin has been demonstrated to inhibit IH in vein grafts in experimental models [25], clinical studies exploring association between statin therapy and vascular access outcomes are controversial [80,81]. A 12-month clinical trial also indicated that neither fish oil which has been proved to be beneficial for AVG [82] nor the antiplatelet agent, aspirin improves AVF patency [83]. In light of the critical role of MR in the pathogenesis of hemodialysis vascular access dysfunction, it is tempting to target MR for preventing or improving AVF non-maturation or failure. MR antagonists (MRAs), like spironolactone and eplerenone, have been applied to cardiovascular diseases with consistent beneficial outcomes [40]. However, the effect of MR blockade on hemodialysis vascular access dysfunction has not been well studied possibly due to a great concern over the potential side effect of hyperkalemia in ESRD patients. With a variable level of residual function, the failed kidney in ESRD patients may still respond to aldosterone and excrete potassium. As such, MRA therapy is likely to diminish renal potassium excretion and cause hyperkalemia. On the other hand, MRAs are able to mitigate potassium disposal in other aldosterone responsive organs, like the intestine and the sweat gland. However, there is evidence suggesting that MRA use in ESRD patients seems to be safe. Indeed, a number of trials recently demonstrated that use of MRAs by ESRD patients did not significantly increase the risk of hyperkalemia but resulted in a better blood pressure control, diminished left ventricular mass, improved left ventricular ejection fraction and reduced mortality rate, in agreement with an overall cardiovascular benefit [84]. Even if MRAs precipitate hyperkalemia in some dialysis patients, new potassium binders, which have been proven by recent clinical trials to effectively lower serum potassium with great tolerance [85], may allow for testing MR blockades in man to preserve AVFs. Moreover, the new generation of nonsteroidal MRAs like finerenone, have a demonstrably improved safety profile and reinforced efficacy than traditional MRAs. Finerenone is more selective for MR than spironolactone and has greater affinity for MR than eplerenone [86]. Thus, nonsteroidal MRAs might provide an opportunity to maximize the beneficial effects of targeting MR in hemodialysis vascular access dysfunction without increasing the risk of hyperkalaemia. Taken together, based on the potential pathogenic role of MR in vascular remodeling, it is conceivable that combined therapy with MR blockades and potassium binders or use of nonsteroidal MRAs in dialysis patients may improve vascular access outcome. This warrants future animal and clinical study to validate the feasibility and efficacy of this therapeutic strategy.
6. Summary and outstanding question
The hemodialysis vascular access is the lifeline for ESRD patients. Vascular access malfunction places substantial clinical, social and economic burden on hemodialysis population. The principal cause of AVF failure is vascular access stenosis histologically characterized by vascular fibrosis and IH. Great efforts have been dedicated to understanding the mechanisms involved in promoting neointima formation and vascular fibrosis. The crucial role of MR in vascular remodeling causing neointima formation and vascuar fibrosis or thrombus is increasingly evident. The MR signaling pathways directly activate VSMC and promote its proliferation, migration, and ECM deposition. MR in endothelial cells impairs NO generation whereas increases production of noxious superoxide. MR signaling in macrophages promotes macrophages differentiation into M1 phenotype and aggravates inflammatory response. All these effects (Fig. 2) act synergistically to promote IH and vascular fibrosis and result in vascular access dysfunction. However, due to the complexity of vascular pathophysiology in ESRD, it is imperative to further decipher the exact role of MR and test the efficacy of MR blockade in hemodialysis vascular access dysfunction in the settings of uremia in future studies. Collectively, therapeutic targeting of MR seems to be a novel and pragmatic modatlity for the prevention and treatment of vascular access dysfunction in hemodialysis patients.
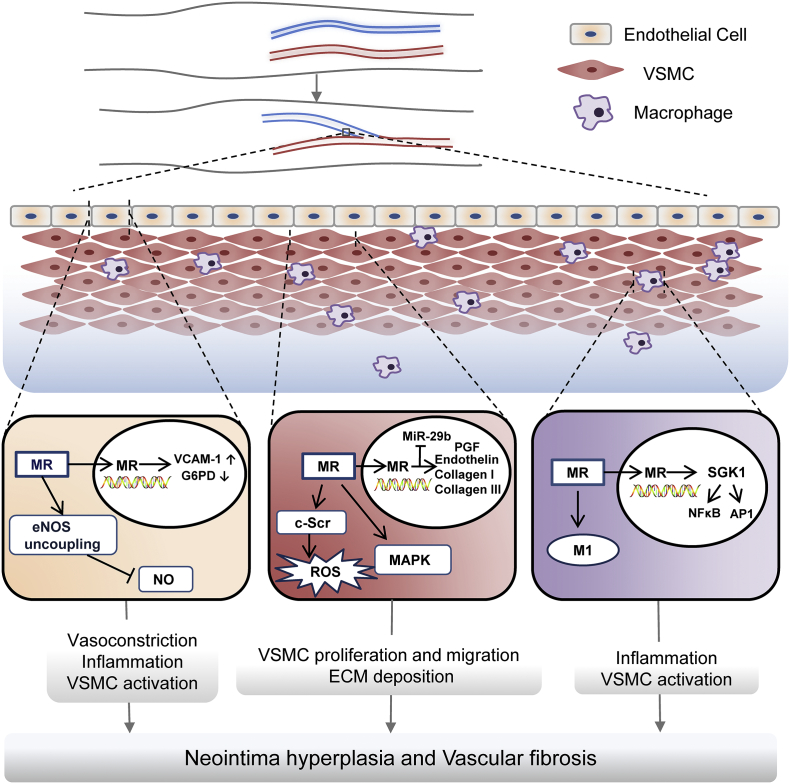
Fig. 2.
Contribution of MR signaling VSMC, endothelial cells and macrophages to vascular access dysfunction. In endothelial cells, MR signaling reduces production and bioavailability of nitric oxide by enhancing eNOS uncoupling, and amplifies inflammation and oxidative stress by promoting endothelial expression of VCAM-1 and inhibiting G6PD, which causes vasoconstriction, inflammation, and VSMC activation. In VSMC, MR exerts non-genomic mineralocorticoid actions, including phosphorylation and activation of MAPK and c-Src, and genomic mineralocorticoid actions, such as upregulation of PGF, endothelin and collagen I/III. In addition, MR signaling may diminish MiR-29b abundance. These effects in VSMC are responsible for VSMC proliferation migration and ECM deposition. In macrophages, MR promotes uncommitted M0 macrophages differentiation to M1 proinflammatory phenotype and activates NFκB and AP1 pathways via SGK1, which can trigger inflammatory response and activate VSMC. All these effects integrate synergistically ultimately resulting in vascular access failure, characterized by neointima hyperplasia and vascular fibrosis. Abbreviations: AP1, activator protein-1; ECM, extracellular matrix; eNOS, endothelial NO synthase; G6PD, glucose-6-phosphate dehydrogenase; MAPK, mitogen-activated protein kinase; MR: mineralocorticoid receptor; NFκB, nuclear factor-Κappa B; PGF, placental growth factor; ROS, reactive oxygen species; SGK-1, serum-and-glucocorticoid-regulated kinase1; VCAM-1, vascular cell adhesion molecule-1; VSMC, vascular smooth muscle cell.
6.1. Search strategy and selection criteria
Data for this review were collected through Pubmed. The following search terms were used: hemodialysis vascular access, arteriovenous fistula (AVF), mineralocorticoid receptor (MR), aldosterone, intimal hyperplasia (IH), vascular smooth muscle cells (VSMC), endothelial cells, and macrophages. Only articles published in English were included.
Acknowledgments
Acknowledgments
This work was supported in part by, the Natural Science Foundation of China grant U1604284, 81670663, 81770672 and 81873612, the 81873612, the Medical Science and Technology Foundation of Henan Province grant 201601002, and the U.S. National Institutes of Health grant DK092485 and DK114006.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
Z.L. and R.G. devised the conceptual ideas. B.C. performed the literature search and drafted the original manuscript. P.W. draw the figures. A.B., L.D. and R.G. contributed to revision. All authors approved the final version of the manuscript.
Contributor Information
Zhangsuo Liu, Email: zhangsuoliu@zzu.edu.cn.
Rujun Gong, Email: Rujun.Gong@UToledo.edu.
References
- 1.Clinical practice guidelines for vascular accessAm J Kidney Dis. 2006;48(Suppl. 1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Bylsma L.C., Gage S.M., Reichert H., Dahl S.L.M., Lawson J.H. Arteriovenous fistulae for haemodialysis: a systematic review and meta-analysis of efficacy and safety outcomes. Eur J Vasc Endovasc Surg. 2017;54(4):513–522. doi: 10.1016/j.ejvs.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Brahmbhatt A., Remuzzi A., Franzoni M., Misra S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int. 2016;89(2):303–316. doi: 10.1016/j.kint.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez L., Duque J.C., Tabbara M. Fibrotic venous remodeling and nonmaturation of arteriovenous fistulas. J Am Soc Nephrol. 2018;29(3):1030–1040. doi: 10.1681/ASN.2017050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupont J.J., Jaffe I.Z. 30 years of the mineralocorticoid receptor: the role of the mineralocorticoid receptor in the vasculature. J Endocrinol. 2017;234(1):T67–t82. doi: 10.1530/JOE-17-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaffe I.Z., Mendelsohn M.E. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96(6):643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 7.Newfell B.G., Iyer L.K., Mohammad N.N. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol. 2011;31(8):1871–1880. doi: 10.1161/ATVBAHA.111.229070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao F., Puddefoot J.R., Vinson G.P. Aldosterone mediates angiotensin II-stimulated rat vascular smooth muscle cell proliferation. J Endocrinol. 2000;165(2):533–536. doi: 10.1677/joe.0.1650533. [DOI] [PubMed] [Google Scholar]
- 9.Bafford R., Sui X.X., Park M. Mineralocorticoid receptor expression in human venous smooth muscle cells: a potential role for aldosterone signaling in vein graft arterialization. Am J Physiol Heart Circ Physiol. 2011;301(1):H41–H47. doi: 10.1152/ajpheart.00637.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P., Brem A.S., Liang X., Ge Y., Liu Z., Gong R. De novo induction of mineralocorticoid receptors in vascular tissue mediates hemodialysis fistula dysfunction. J Am Soc Nephrol. 2016;352A(27) Abstract Edition. [Google Scholar]
- 11.Ehsan A., McGraw A.P., Aronovitz M.J. Mineralocorticoid receptor antagonism inhibits vein graft remodeling in mice. J Thorac Cardiovasc Surg. 2013;145(6):1642–1649. doi: 10.1016/j.jtcvs.2012.08.007. 9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbin M.L., Greene T., Cheung A.K. Arteriovenous fistula development in the first 6 weeks after creation. Radiology. 2016;279(2):620–629. doi: 10.1148/radiol.2015150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remuzzi A., Ene-Iordache B. Novel paradigms for dialysis vascular access: upstream hemodynamics and vascular remodeling in dialysis access stenosis. Clin J Am Soc Nephrol. 2013;8(12):2186–2193. doi: 10.2215/CJN.03450413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy-Chaudhury P., Sukhatme V.P., Cheung A.K. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17(4):1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez-Padron R.I., Allon M. new insights into dialysis vascular access: impact of preexisting arterial and venous pathology on AVF and AVG outcomes. Clin J Am Soc Nephrol. 2016;11(8):1495–1503. doi: 10.2215/CJN.01860216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung A.K., Imrey P.B., Alpers C.E. Intimal hyperplasia, stenosis, and arteriovenous fistula maturation failure in the hemodialysis fistula maturation study. J Am Soc Nephrol. 2017;28(10):3005–3013. doi: 10.1681/ASN.2016121355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabbara M., Duque J.C., Martinez L. Pre-existing and postoperative intimal hyperplasia and arteriovenous fistula outcomes. Am J Kidney Dis. 2016;68(3):455–464. doi: 10.1053/j.ajkd.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duque J.C., Tabbara M., Martinez L. Similar degree of intimal hyperplasia in surgically detected stenotic and nonstenotic arteriovenous fistula segments: a preliminary report. Surgery. 2018;163(4):866–869. doi: 10.1016/j.surg.2017.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy-Chaudhury P., Wang Y., Krishnamoorthy M. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant. 2009;24(9):2786–2791. doi: 10.1093/ndt/gfn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall M.R., Yamamoto K., Protack C.D. Temporal regulation of venous extracellular matrix components during arteriovenous fistula maturation. J Vasc Access. 2015;16(2):93–106. doi: 10.5301/jva.5000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis M.E., Cai H., Drummond G.R., Harrison D.G. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res. 2001;89(11):1073–1080. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- 22.Marin M.L., Veith F.J., Panetta T.F. Saphenous vein biopsy: a predictor of vein graft failure. J Vasc Surg. 1993;18(3):407–414. [discussion 14-5] [PubMed] [Google Scholar]
- 23.Liang A., Wang Y., Han G., Truong L., Cheng J. Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am J Physiol Renal Physiol. 2013;304(12):F1413–F1420. doi: 10.1152/ajprenal.00585.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwahara G., Hashimoto T., Tsuneki M. CD44 promotes inflammation and extracellular matrix production during arteriovenous fistula maturation. Arterioscler Thromb Vasc Biol. 2017;37(6):1147–1156. doi: 10.1161/ATVBAHA.117.309385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Jin H., Huang J. Local delivery of pravastatin inhibits intimal formation in a mouse vein graft model. Can J Cardiol. 2012;28(6):750–757. doi: 10.1016/j.cjca.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Rammos C., Hendgen-Cotta U.B., Sobierajski J. Macrophage migration inhibitory factor is associated with vascular dysfunction in patients with end-stage renal disease. Int J Cardiol. 2013;168(6):5249–5256. doi: 10.1016/j.ijcard.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Misra S., Fu A.A., Rajan D.K. Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. J Vasc Interven Radiol. 2008;19(2 Pt 1):252–259. doi: 10.1016/j.jvir.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Rothuizen T.C., Wong C., Quax P.H., van Zonneveld A.J., Rabelink T.J., Rotmans J.I. Arteriovenous access failure: more than just intimal hyperplasia? Nephrol Dial Transplant. 2013;28(5):1085–1092. doi: 10.1093/ndt/gft068. [DOI] [PubMed] [Google Scholar]
- 29.Lee E.S., Shen Q., Pitts R.L. Serum metalloproteinases MMP-2, MMP-9, and metalloproteinase tissue inhibitors in patients are associated with arteriovenous fistula maturation. J Vasc Surg. 2011;54(2):454–459. doi: 10.1016/j.jvs.2011.02.056. [discussion 9-60] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tronc F., Mallat Z., Lehoux S., Wassef M., Esposito B., Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20(12):E120–E126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- 31.Allon M., Greene T., Dember L.M. Association between preoperative vascular function and postoperative arteriovenous fistula development. J Am Soc Nephrol. 2016;27(12):3788–3795. doi: 10.1681/ASN.2015020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simone S., Loverre A., Cariello M. Arteriovenous fistula stenosis in hemodialysis patients is characterized by an increased adventitial fibrosis. J Nephrol. 2014;27(5):555–562. doi: 10.1007/s40620-014-0050-7. [DOI] [PubMed] [Google Scholar]
- 33.Bruner K.L., Derfoul A., Robertson N.M. The unliganded mineralocorticoid receptor is associated with heat shock proteins 70 and 90 and the immunophilin FKBP-52. Recept Signal Transduct. 1997;7(2):85–98. [PubMed] [Google Scholar]
- 34.Brem A.S., Gong R. Therapeutic targeting of aldosterone: a novel approach to the treatment of glomerular disease. Clin Sci (Lond) 2015;128(9):527–535. doi: 10.1042/CS20140432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu M., Wang P., Ge Y. Activation of mineralocorticoid receptor by ecdysone, an adaptogenic and anabolic ecdysteroid, promotes glomerular injury and proteinuria involving overactive GSK3beta pathway signaling. Sci Rep. 2018;8(1):12225. doi: 10.1038/s41598-018-29483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagase M., Fujita T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol. 2013;9(2):86–98. doi: 10.1038/nrneph.2012.282. [DOI] [PubMed] [Google Scholar]
- 37.Lombes M., Oblin M.E., Gasc J.M., Baulieu E.E., Farman N., Bonvalet J.P. Immunohistochemical and biochemical evidence for a cardiovascular mineralocorticoid receptor. Circ Res. 1992;71(3):503–510. doi: 10.1161/01.res.71.3.503. [DOI] [PubMed] [Google Scholar]
- 38.Brem A.S., Morris D.J., Li X., Ge Y., Shaw S., Gong R. Adrenalectomy amplifies aldosterone induced injury in cardiovascular tissue: an effect attenuated by adrenally derived steroids. Steroids. 2013;78(3):347–355. doi: 10.1016/j.steroids.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Pandey A., Garg S., Matulevicius S.A. Effect of mineralocorticoid receptor antagonists on cardiac structure and function in patients with diastolic dysfunction and heart failure with preserved ejection fraction: a meta-analysis and systematic review. J Am Heart Assoc. 2015;4(10) doi: 10.1161/JAHA.115.002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zannad F., McMurray J.J., Krum H. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 41.Wakabayashi K., Suzuki H., Sato T., Iso Y., Katagiri T., Takeyama Y. Eplerenone suppresses neointimal formation after coronary stent implantation in swine. Int J Cardiol. 2006;107(2):260–266. doi: 10.1016/j.ijcard.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 42.Ward M.R., Kanellakis P., Ramsey D., Funder J., Bobik A. Eplerenone suppresses constrictive remodeling and collagen accumulation after angioplasty in porcine coronary arteries. Circulation. 2001;104(4):467–472. doi: 10.1161/hc3001.091458. [DOI] [PubMed] [Google Scholar]
- 43.Dutzmann J., Musmann R.J., Haertle M. The novel mineralocorticoid receptor antagonist finerenone attenuates neointima formation after vascular injury. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey A.P., Montezano A.C., Hood K.Y. Vascular dysfunction and fibrosis in stroke-prone spontaneously hypertensive rats: the aldosterone-mineralocorticoid receptor-Nox1 axis. Life Sci. 2017;179:110–119. doi: 10.1016/j.lfs.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9(8):459–469. doi: 10.1038/nrneph.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiffrin E.L., Franks D.J., Gutkowska J. Effect of aldosterone on vascular angiotensin II receptors in the rat. Can J Physiol Pharmacol. 1985;63(12):1522–1527. doi: 10.1139/y85-250. [DOI] [PubMed] [Google Scholar]
- 47.Briet M., Barhoumi T., Mian M.O.R. Aldosterone-induced vascular remodeling and endothelial dysfunction require functional angiotensin type 1a receptors. Hypertension (Dallas, Tex : 1979) 2016;67(5):897–905. doi: 10.1161/HYPERTENSIONAHA.115.07074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y.W., Wu Y.T., Lin J.S. Association of genetic polymorphisms of renin-angiotensin-aldosterone system-related genes with arterio-venous fistula malfunction in hemodialysis patients. Int J Mol Sci. 2016:17(6). doi: 10.3390/ijms17060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moon J.Y., Jeong K.H., Paik S.S. Arteriovenous fistula patency associated with angiotensin-converting enzyme I/D polymorphism and ACE inhibition or AT1 receptor blockade. Nephron Clin Pract. 2009;111(2):c110–c116. doi: 10.1159/000191201. [DOI] [PubMed] [Google Scholar]
- 50.Sajgure A., Choudhury A., Ahmed Z., Choudhury D. Angiotensin converting enzyme inhibitors maintain polytetrafluroethylene graft patency. Nephrol Dial Transplant. 2007;22(5):1390–1398. doi: 10.1093/ndt/gfl821. [DOI] [PubMed] [Google Scholar]
- 51.Lin C.C., Yang W.C. Prognostic factors influencing the patency of hemodialysis vascular access: literature review and novel therapeutic modality by far infrared therapy. J Chin Med Assoc. 2009;72(3):109–116. doi: 10.1016/S1726-4901(09)70035-8. [DOI] [PubMed] [Google Scholar]
- 52.Lee T., Wang Y., Arend L. Comparative analysis of cellular phenotypes within the neointima from vein segments collected prior to vascular access surgery and stenotic arteriovenous dialysis accesses. Semin Dial. 2014;27(3):303–309. doi: 10.1111/sdi.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wasse H., Huang R., Naqvi N., Smith E., Wang D., Husain A. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. J Vasc Access. 2012;13(2):168–174. doi: 10.5301/jva.5000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Senthoor D., Thant K.Z., Ng T.K., Ho P. Clinical course of hemodialysis access after initial endovascular intervention for stenosis in Asian renal failure patients. Vasc Endovascular Surg. 2017;51(6):363–367. doi: 10.1177/1538574417706639. [DOI] [PubMed] [Google Scholar]
- 55.Pruthi D., McCurley A., Aronovitz M., Galayda C., Karumanchi S.A., Jaffe I.Z. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34(2):355–364. doi: 10.1161/ATVBAHA.113.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCurley A., Pires P.W., Bender S.B. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18(9):1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koenig J.B., Jaffe I.Z. Direct role for smooth muscle cell mineralocorticoid receptors in vascular remodeling: novel mechanisms and clinical implications. Curr Hypertens Rep. 2014;16(5):427. doi: 10.1007/s11906-014-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bretschneider M., Busch B., Mueller D. Activated mineralocorticoid receptor regulates micro-RNA-29b in vascular smooth muscle cells. FASEB J. 2016;30(4):1610–1622. doi: 10.1096/fj.15-271254. [DOI] [PubMed] [Google Scholar]
- 59.Mills C.D. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32(6):463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 60.Kondo Y., Jadlowiec C.C., Muto A. The Nogo-B-PirB axis controls macrophage-mediated vascular remodeling. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0081019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Usher M.G., Duan S.Z., Ivaschenko C.Y. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120(9):3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun J.Y., Li C., Shen Z.X. Mineralocorticoid receptor deficiency in macrophages inhibits neointimal hyperplasia and suppresses macrophage inflammation through SGK1-AP1/NF-kappaB pathways. Arterioscler Thromb Vasc Biol. 2016;36(5):874–885. doi: 10.1161/ATVBAHA.115.307031. [DOI] [PubMed] [Google Scholar]
- 63.Bhargava A., Fullerton M.J., Myles K. The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology. 2001;142(4):1587–1594. doi: 10.1210/endo.142.4.8095. [DOI] [PubMed] [Google Scholar]
- 64.Cheng J., Wang Y., Ma Y. The mechanical stress-activated serum-, glucocorticoid-regulated kinase 1 contributes to neointima formation in vein grafts. Circ Res. 2010;107(10):1265–1274. doi: 10.1161/CIRCRESAHA.110.222588. [DOI] [PubMed] [Google Scholar]
- 65.Borst O., Schaub M., Walker B. Pivotal role of serum- and glucocorticoid-inducible kinase 1 in vascular inflammation and atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35(3):547–557. doi: 10.1161/ATVBAHA.114.304454. [DOI] [PubMed] [Google Scholar]
- 66.Yamasaki K., Asai T., Shimizu M. Inhibition of NFkappaB activation using cis-element ‘decoy’ of NFkappaB binding site reduces neointimal formation in porcine balloon-injured coronary artery model. Gene Ther. 2003;10(4):356–364. doi: 10.1038/sj.gt.3301875. [DOI] [PubMed] [Google Scholar]
- 67.Weiss M.F., Scivittaro V., Anderson J.M. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis. 2001;37(5):970–980. doi: 10.1016/s0272-6386(05)80013-7. [DOI] [PubMed] [Google Scholar]
- 68.Katusic Z.S., D'Uscio L.V., Nath K.A. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci. 2009;30(1):48–54. doi: 10.1016/j.tips.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Victorio J.A., Clerici S.P., Palacios R. Spironolactone prevents endothelial nitric oxide synthase uncoupling and vascular dysfunction induced by beta-adrenergic overstimulation: role of perivascular adipose tissue. Hypertension (Dallas, Tex : 1979) 2016;68(3):726–735. doi: 10.1161/HYPERTENSIONAHA.116.07911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kratz M.T., Schirmer S.H., Baumhakel M., Bohm M. Improvement of endothelial function in a murine model of mild cholesterol-induced atherosclerosis by mineralocorticoid antagonism. Atherosclerosis. 2016;251:291–298. doi: 10.1016/j.atherosclerosis.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 71.Leopold J.A., Dam A., Maron B.A. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13(2):189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dinh Q.N., Young M.J., Evans M.A., Drummond G.R., Sobey C.G., Chrissobolis S. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res. 1637;2016:146–153. doi: 10.1016/j.brainres.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 73.Davel A.P., Anwar I.J., Jaffe I.Z. The endothelial mineralocorticoid receptor: mediator of the switch from vascular health to disease. Curr Opin Nephrol Hypertens. 2017;26(2):97–104. doi: 10.1097/MNH.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bodary P.F., Sambaziotis C., Wickenheiser K.J., Rajagopalan S., Pitt B., Eitzman D.T. Aldosterone promotes thrombosis formation after arterial injury in mice. Arterioscler Thromb Vasc Biol. 2006;26(1):233. doi: 10.1161/01.ATV.0000195782.07637.44. [DOI] [PubMed] [Google Scholar]
- 75.Lagrange J., Li Z., Fassot C. Endothelial mineralocorticoid receptor activation enhances endothelial protein C receptor and decreases vascular thrombosis in mice. FASEB J. 2014;28(5):2062–2072. doi: 10.1096/fj.13-238188. [DOI] [PubMed] [Google Scholar]
- 76.Deuchar G.A., McLean D., Hadoke P.W.F. 11beta-hydroxysteroid dehydrogenase type 2 deficiency accelerates atherogenesis and causes proinflammatory changes in the endothelium in apoe−/− mice. Endocrinology. 2011;152(1):236–246. doi: 10.1210/en.2010-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang C.J., Ko Y.S., Ko P.J. Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney Int. 2005;68(3):1312–1319. doi: 10.1111/j.1523-1755.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 78.Fadel F.I., Elshamaa M.F., Nabhan M.M. Soluble adhesion molecules as markers of native arteriovenous fistula thrombosis in children on uremia. Blood Coagulat Fibrinol. 2014;25(7):675–682. doi: 10.1097/MBC.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 79.Lin C.C., Yang W.C., Chen M.C., Liu W.S., Yang C.Y., Lee P.C. Effect of far infrared therapy on arteriovenous fistula maturation: an open-label randomized controlled trial. Am J Kidney Dis. 2013;62(2):304–311. doi: 10.1053/j.ajkd.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 80.Pisoni R., Barker-Finkel J., Allo M. Statin therapy is not associated with improved vascular access outcomes. Clin J Am Soc Nephrol. 2010;5(8):1447–1450. doi: 10.2215/CJN.02740310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang H.H., Chang Y.K., Lu C.W. Statins improve long term patency of arteriovenous fistula for hemodialysis. Sci Rep. 2016;6:22197. doi: 10.1038/srep22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lok C.E., Moist L., Hemmelgarn B.R. Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: a randomized controlled trial. JAMA. 2012;307(17):1809–1816. doi: 10.1001/jama.2012.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Irish A.B., Viecelli A.K., Hawley C.M. Effect of fish oil supplementation and aspirin use on arteriovenous fistula failure in patients requiring hemodialysis: a randomized clinical trial. JAMA Intern Med. 2017;177(2):184–193. doi: 10.1001/jamainternmed.2016.8029. [DOI] [PubMed] [Google Scholar]
- 84.Bomback A.S. Mineralocorticoid Receptor Antagonists in End-Stage Renal Disease: Efficacy and Safety. Blood Purif. 2016;41(1–3):166–170. doi: 10.1159/000441262. [DOI] [PubMed] [Google Scholar]
- 85.Pitt B., Bakris G.L. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension (Dallas, Tex : 1979) 2015;66(4):731–738. doi: 10.1161/HYPERTENSIONAHA.115.04889. [DOI] [PubMed] [Google Scholar]
- 86.Dojki F.K., Bakris G. Nonsteroidal mineralocorticoid antagonists in diabetic kidney disease. Curr Opin Nephrol Hypertens. 2017;26(5):368–374. doi: 10.1097/MNH.0000000000000340. [DOI] [PubMed] [Google Scholar]