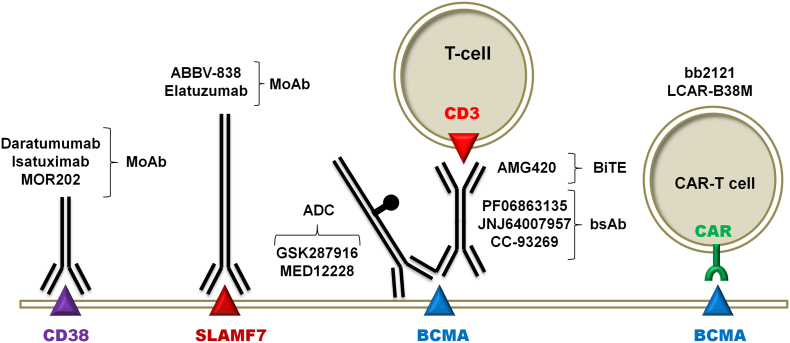
Fig. 1.
Effective Biotherapy Targets on the Surface of Multiple Myeloma Cells. Abbreviations: MoAb: monoclonal antibody; BiTE: bispecific T-cell engager; ADC: antibody-drug conjugate; bsAb: bispecific antibody; CAR-T: chimeric antigen receptor carrying T-cell. There are 2 ADCs targeting BCMA in clinical development, namely GSK2857916 and MEDI2228. GSK2857916 is a humanized anti-BCMA MoAb conjugated to the cytotoxic agent monomethyl auristatin-F, via the non-cleavable linker maleimidocaproyl. GSK2857916 monotherapy has demonstrated a 60% ORR and a median PFS of 7.9 months in a group of hard to treat and heavily pretreated R/R MM [18]. It has recently received Breakthrough Therapy designation from FDA and also received PRIME designation from the European Medicines Agency (EMA). MEDI2228, a fully human MoAb that is conjugated to pyrrolobenzodiazepine dimer via a protease-cleavable linker is being evaluated in the Phase 1 study NCT03489525 for the treatment of MM. BiTEs are composed of two single-chain variable fragments (scFvs) connected by a flexible linker. One scFv fragment binds to a T cell-specific antigen (typically CD3), whereas the other scFv fragment binds to a tumor-specific antigen. This bispecificity allows BiTEs to juxtapose T-cells and tumor cells physically and promotes the formation of immunological synapses by the simultaneous binding of multiple BiTEs, leading to T-cell activation, cytokine production and cytotoxicity of the tumor cells. BI 836909 (AMG 420) is a BiTE targeting BCMA and CD3ɛ. BsAbs are a class of engineered antibody and antibody-like proteins that, in contrast to ‘regular’ monospecific antibodies, combine two or more different specific antigen binding elements in a single construct. Since bsAbs do not typically occur in nature, they are constructed either chemically or biologically, using techniques such as cell fusion or recombinant DNA technologies. There are 3 clinical stage anti-CD3xBCMA bsAbs, namely Johnson and Johnson's JNJ64007957 (NCT03145181), Pfizer's PF-06863135 (NCT03269136), and Celgene's CC-93269/EM901 (NCT03486067) that have entered Phase 1 testing. See the text for a detailed discussion of the CAR-T cell platforms.