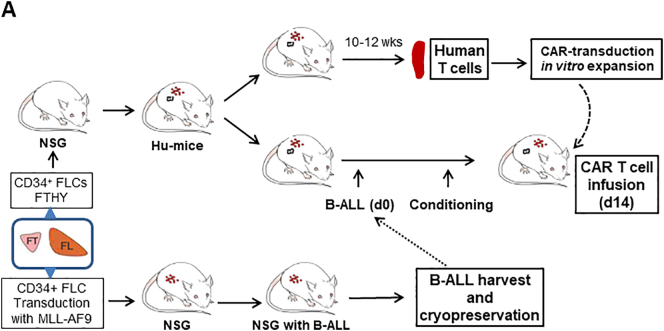
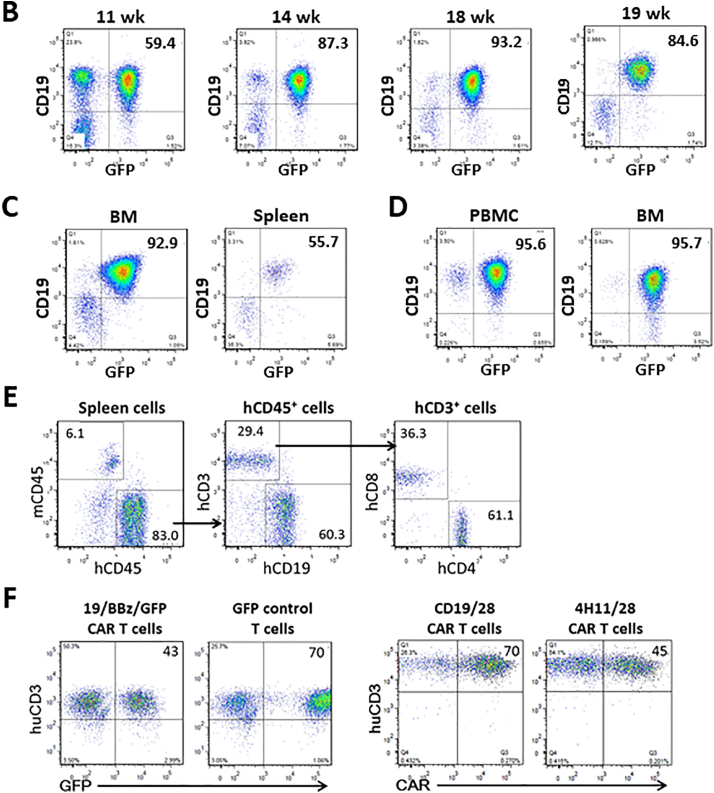
Fig. 1.
Modeling anti-CD19 CAR T cell therapy in humanized mice without allogeneic or xenogeneic immune responses. (A) Schematic outline of the experimental design. (B—C) Representative FACS profiles showing the presence of human CD19+GFP+ leukemia cells in blood (PBMC) at the indicated times (B), and in the bone marrow (BM) and spleen at week 19 (C) from NSG mice receiving MLL-AF9-transduced CD34+ FLCs. (D) FACS profiles showing the presence of human CD19+GFP+ leukemia cells in PBMC and BM from a representative NSG mouse receiving GFP+ leukemia cells from the primary leukemic mice 15 weeks after adoptive transfer. (E-F) For each experiment, we sacrificed 2 hu-mice for generating CAR T cells. From two hu-mice we could obtain 1.5 × 107 or more human CD3+ T cells, and from which we could generate >15 × 107 CAR T cells after transduction and expansion (sufficient for >30 mice at the dose of 5 × 106 per mouse). Human CD3+ T cells were isolated from hu-mouse spleens by FACS and transduced with anti-CD19 CAR or control viruses. (E) FACS profiles showing human immune cell chimerism in the spleens of hu-mice. (F) Transduced hu-mouse splenic CD3+ T cells were expanded in vitro for 2 weeks and analyzed for the expression of 19/BBz/GFP CAR and GFP (Left), or CD19/28 CAR and control 4H11/28 CAR (Right). Numbers on the figure indicate the percentages of positively-stained cells.