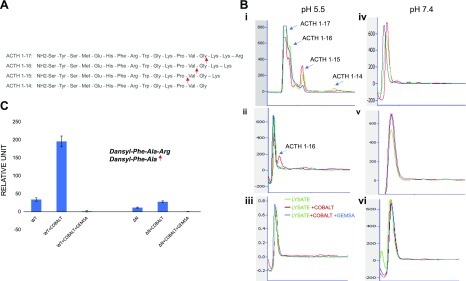
Figure 3.
The enzymatic activity of CPE-ΔN was analyzed by HPLC. A) Sequence of ACTH (1–17)–(1–14) showing cleavage sites for CPE at C-terminal basic amino acid residues. B) Lysates from CPE-WT, CPE-ΔN, or control adenovirus-infected COS-7 cells were incubated for 24 h with ACTH (1–17), with and without cobalt and/or GEMSA, and the enzymatically processed products were analyzed by HPLC. At pH 5.5, CPE-WT generated different peptide products: ACTH (1–16), ACTH (1–15), and ACTH (1–14), which were induced by cobalt and inhibited by GEMSA (i). CPE-ΔN generated only 1 peptide product, ACTH (1–16) (ii); whereas control lysate did not show any peptide product (iii). At pH 7.4, no product peaks were observed for any lysate from CPE-WT (iv), ΔN–CPE (v), or control adenovirus-infected COS-7 cells (vi). C) Enzymatic activity of purified CPE-WT or CPE-ΔN was measured with dansyl-Phe-Ala-Arg as the substrate. CPE-WT (40 ng) and CPE-ΔN (100 ng) were incubated with dansyl-Phe-Ala-Arg (0.5 mM) for 16 h at 37°C in the presence or absence of CoCl2 and GEMSA, and fluorescence was recorded as excitation (360/40 nm filter) and emission (528/20 nm filter). Values shown in bar graph are corrected for 100-ng protein (CPE-WT). means ± sd, n = 2.