Figure 5.
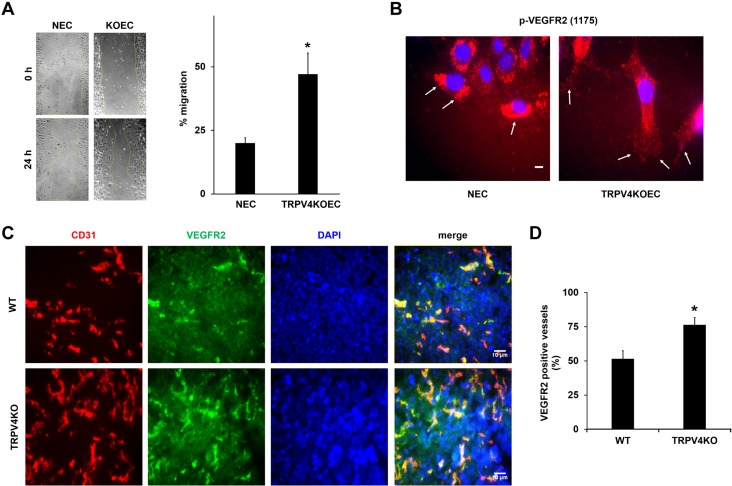
Deletion of TRPV4 increases VEGF-induced migration in vitro and VEGFR2-positive vessels in tumors in vivo. A) Migration of serum-starved NECs and TRPV4KOECs was measured by using a scratch-wound migration assay. The graph represents the percent migration of NECs and TRPV4KOECs. B) Immunofluorescence images of p-VEGFR2 (red) in migrating NECs and TRPV4KOECs. Arrows depict membrane localization of p-VEGFR2 in ECs at the wound edge. Nuclei were stained with DAPI (blue). C) Tumors were implanted into WT and TRPV4KO mice by subcutaneously injecting Lewis lung carcinoma cells, as previously described (13), and isolated on day 21. Representative images (20×) of the tumor tissue stained with CD31 (red), VEGFR2 (green), and DAPI (nuclei) were used to quantify the VEGFR2-positive vessels. D) Quantitative analysis demonstrating a significant increase in the percentage of VEGFR2-positive vessels in tumors from TRPV4KO mice compared with WT mice. Data presented are means ± sem from 3 independent experiments. *P ≤ 0.05. Scale bars, 10 μm.