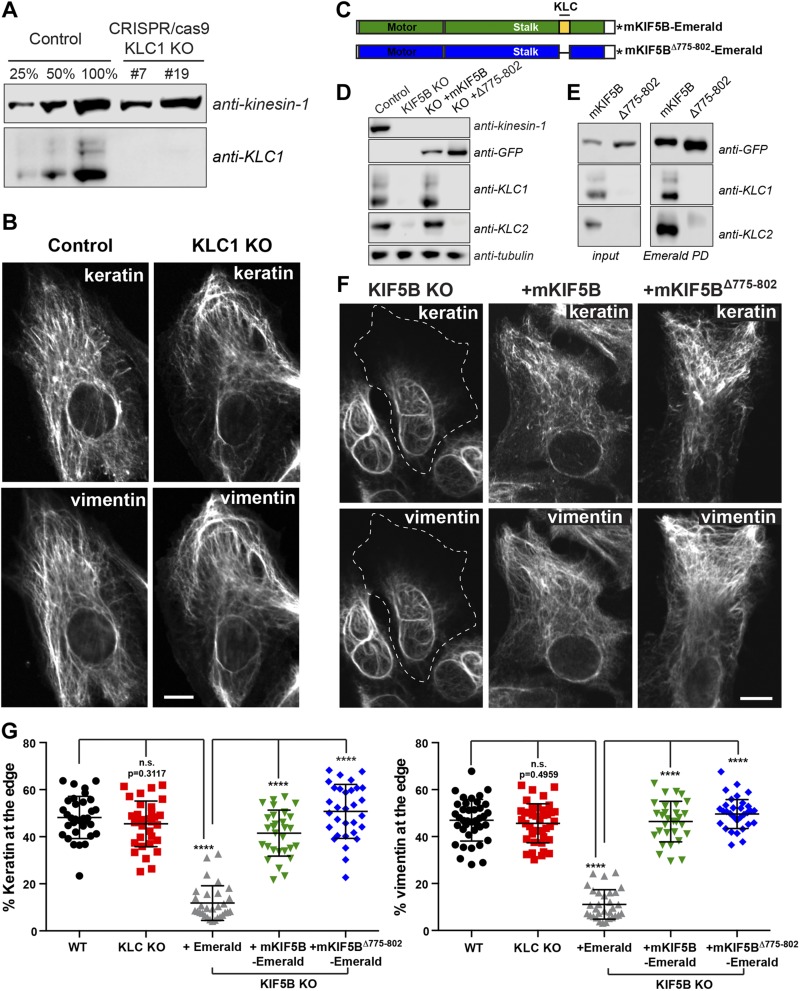
Figure 4.
Transport of keratin and vimentin filaments is independent of kinesin light chain. A) Western blot analyses using KLC-1 antibody showed the absence of KLC-1 in 2 different KLC-1 CRISPR clones (#7 and #19). KIF5 antibody was used as loading control. B) Confocal microscopy of keratin and vimentin immunostaining in RPE cells WT (control) and KLC-1 KO (clone #7). C) Schematic representation of mKIF5B-Emerald and mKIF5BΔ775–802-Emerald. The domain in blue is the motor domain of mKIF5B, and the red part is the stalk, which is comprised of the heptad repeat domain responsible for KLC binding in yellow. This KLC binding domain is absent from the mKIF5BΔ775–802-Emerald. The asterisk represents Emerald. D) Western blot analyses of kinesin-1, KLC1, and KLC2 showed that KLCs were absent from the lysate prepared from the KIF5B KO cells. Antitubulin was used as loading control. E) Endogenous KIF5B was replaced by mKIF5B-Emerald or mKIF5BΔ775–802-Emerald in RPE KIF5B KO #4. The presence of KLC1 and KLC2 was determined by Western blot of crude cell lysates (left panel) or after enrichment of the kinesin-1 complex by pulldown using GFP-binder recognizing Emerald. F) Confocal microscopy imaging of keratin and vimentin immunostaining in RPE KIF5B KO #4 cells after the expression of Emerald (KIF5B KO), mKIF5B-Emerald (+mKIF5B), or mKIF5BΔ775–802-Emerald (+Δ775–802). G) Graphs show the percentage of vimentin (left) or keratin (right) at the cell edge (mean with sd; n > 30 cells). Data are representative of at least 2 independent experiments. Statistical significance was determined using the Mann-Whitney U test. ****P < 0.0001.