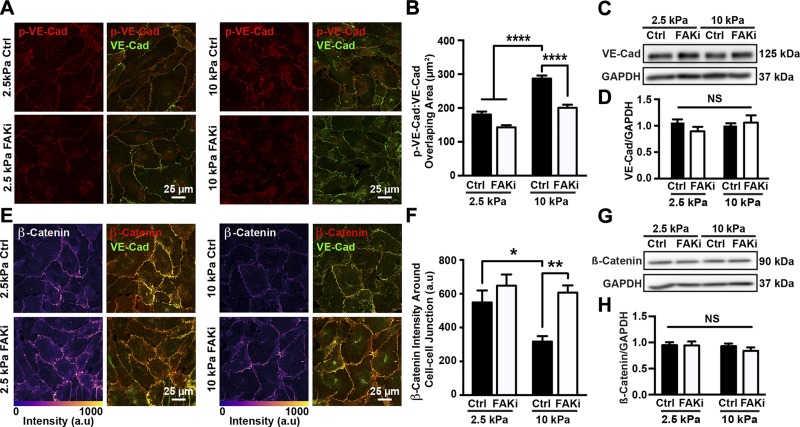
Figure 3.
Matrix stiffness regulates VE-cadherin phosphorylation and adherens junction architecture through FAK activation. A) Representative confocal microscopy images of HUVECs seeded on compliant (2.5 kPa) or stiff (10 kPa) substrates, treated with (FAKi) or without (Ctrl) PF573228. Cells were stained for VE-cadherin (VE-Cad) and phosphorylated VE-cadherin (p-VE-Cad). B) Corresponding quantification of overlapping area of phosphorylated VE-cadherin and VE-cadherin showing that matrix stiffening increased amount of phosphorylated VE-cadherin around adherens junctions, while FAK inhibition restored phosphorylated VE-cadherin to levels on compliant substrates (n = 3; 25–35 cells were included in each condition for each experiment). C) Protein bands generated by Western blot showing VE-cadherin. GAPDH was utilized as loading control; 2.5 and 10 kPa indicate stiffness of PA gel substrate. Ctrl indicates samples treated with vehicle solution; FAKi, samples treated with FAK inhibitor PF573228. D) Corresponding quantification of VE-cadherin normalized over GAPDH (n = 6). E) Confocal microscopy images showing HUVECs seeded on compliant (2.5 kPa) or stiff (10 kPa) PA gel substrates, treated with or without PF573228. Cells were stained for VE-cadherin and β-catenin. F) Corresponding quantification of β-catenin at adherens junctions showing increased substrate stiffness increased amount of β-catenin around junctions (n = 3; 20–30 cells were included in each condition during each experiment). G) Protein bands generated with Western blot showing β-catenin expression. H) Corresponding quantification of β-catenin normalized over GAPDH (n = 6). Data are presented as means ± se. NS, no statistical difference. ****P < 0.0001, **P = 0.0011, *P < 0.005.