Figure 1. Developing neoantigen-specific T cells by reverse immunology.
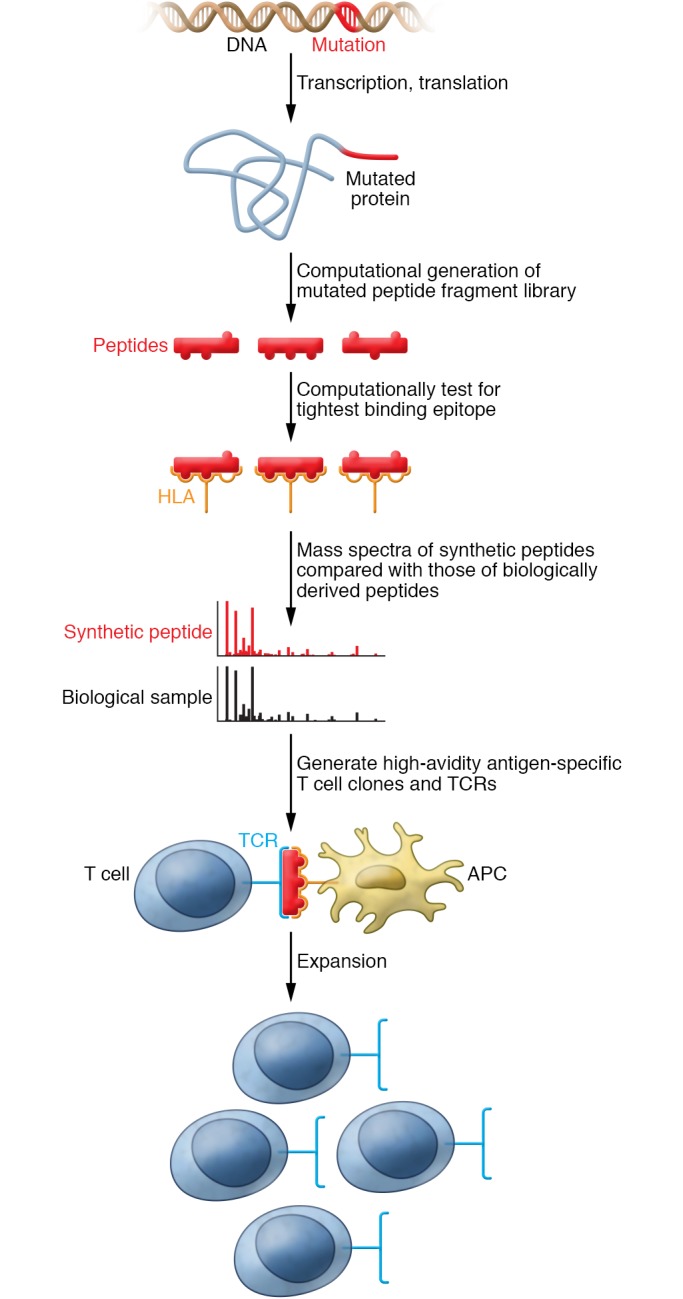
For a “public” neoantigen, a common mutation is identified. In the case of NPM1, this is a 4 base pair insertion. The translational result of the mutation is predicted, which for NPM1 is an out of frame amino acid sequence at the C terminus. The new peptide sequences are tested for predicted binding to an HLA type of interest to determine the most likely neoepitopes. Targeted mass spectrometry is performed to compare peptide epitope sequences from a biological sample (e.g., cell line or primary tumor) to a synthetic peptide standard. High-avidity antigen-specific T cell clones are generated, and their T cell receptors are sequenced. The T cell receptor is incorporated into a viral vector into subsequent generation of T cell populations for use in ACT.
