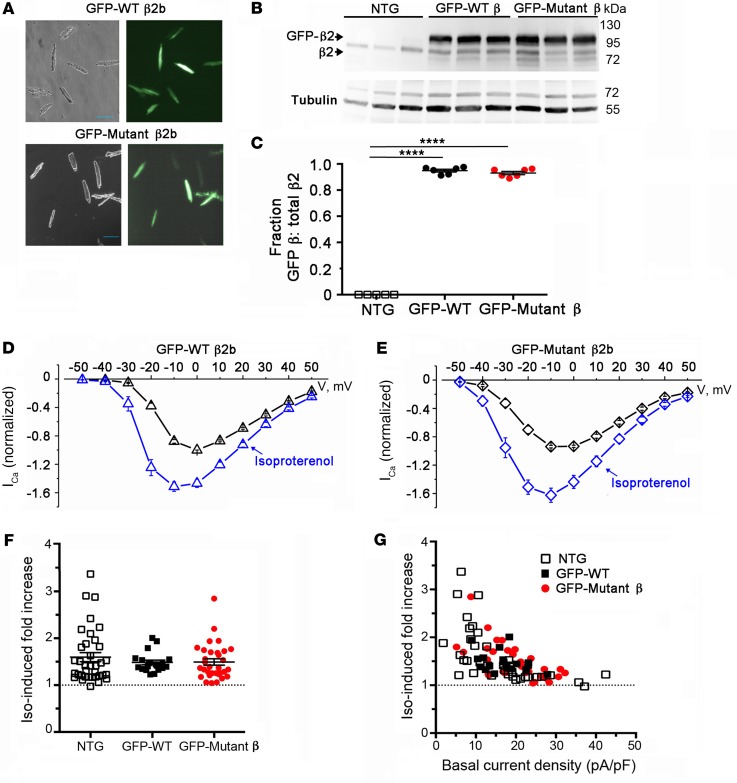
Figure 4. PKA phosphorylation of CaV β is not required for β-adrenergic regulation of CaV1.2. (A) Bright-field and GFP image of WT and mutant β2b-expressing cardiomyocytes.
Scale bars: 100 μm. (B) Immunoblots using anti–β2 antibody (upper) and anti–tubulin antibody of homogenates from the hearts of nontransgenic (NTG) and doxycycline-fed GFP-WT β2 and GFP-mutant β2-expressing mice. (C) Graph of densitometry of fraction of GFP-β/total β. Mean ± SEM; n = 6 mice for NTG, WT, and mutant β2. ****P < 0.0001 compared with nontransgenic by 1-way ANOVA and Dunnett’s multiple comparison test. (D and E) Normalized current-voltage relationships of GFP-WT β2 and GFP-mutant β2 cardiomyocytes acquired before and after superfusion of 200 nM isoproterenol. Isoproterenol shifted the Vmid of steady-state activation of GFP-WT β2 and GFP-mutant β2 cardiomyocytes by –7.0 mV (P < 0.0001, t test, n = 15) and –7.5 mV (P < 0.001, t test, n = 30), respectively. (F) Column scatter plot depicting the fold increase in peak current caused by isoproterenol. Mean ± SEM; n = 36 cardiomyocytes from 5 NTG mice; n = 19 cardiomyocytes from 4 GFP-WT β2b mice; n = 32 cardiomyocytes from 5 mutant β2b mice. P = 0.55 by 1-way ANOVA. (G) Graphs of isoproterenol-induced increase in current stratified by total basal current density for cardiomyocytes isolated from NTG mice, GFP-WT β2b mice, and GFP-mutant β2b transgenic mice.