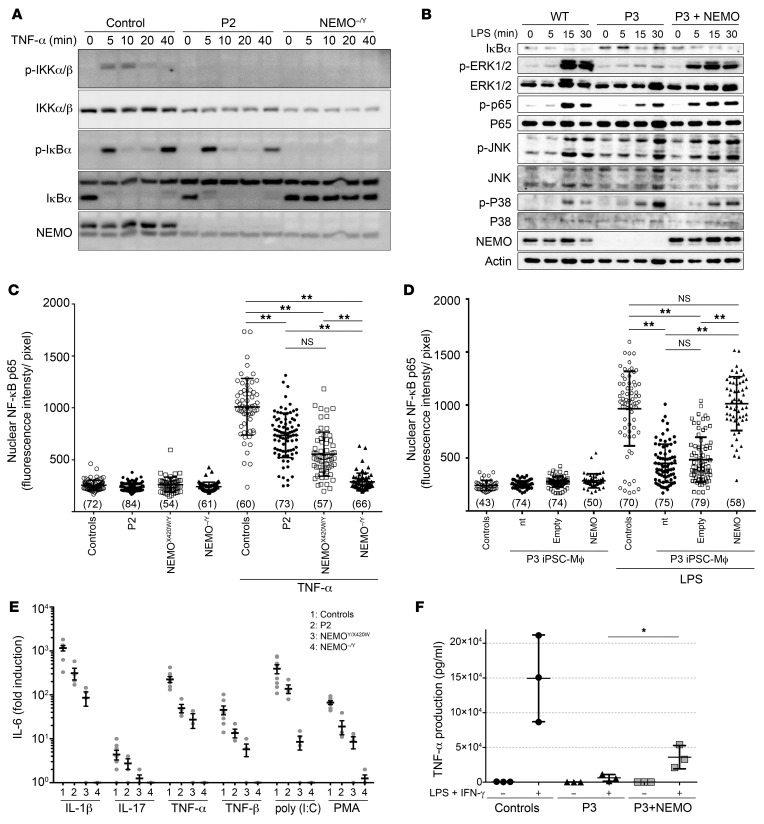
Figure 6. NEMO insufficiency impairs NF‑κB activation in patients’ fibroblasts and iPSC-derived Mϕ.
(A) Impaired NF-κB activation in response to TNF-α. Time course for TNF-α–stimulated SV40‑immortalized fibroblasts, showing impaired IKKα/β phosphorylation (p-IKKα/β) but no impairment of IκBα degradation in P2 relative to the control. (B) Impaired NF-κB and ERK activation in response to LPS in P3-derived iPSC-Mϕ. Time course for LPS-stimulated iPSC-Mϕ, showing impaired phosphorylation of p65 and ERK1/2, but not of p38 or JNK, which are 2 proteins activated independently of NEMO. NF‑κB and ERK activation was rescued by reexpression of WT NEMO (P3 + NEMO). (C and D) Impaired NF-κB p65 nuclear translocation (C) in P2-derived SV40-immortalized fibroblasts and (D) P3-derived iPSC-Mϕ. NF-κB p65 nuclear translocation was rescued by reexpression of WT NEMO (P3 + NEMO). Data are shown as the mean ± SD of cells from more than 4 random fields taken from 1 well per condition, and the number of cells analyzed in each condition is shown in parentheses. Representative results of 3 independent experiments are shown. **P < 0.001, by 1-way ANOVA followed by Turkey’s test for multiple comparisons. (E) Impaired IL-6 production in response to TNF-α, TNF-β, IL-1β, poly (I:C), IL-17, and PMA in SV40-immortalized fibroblasts from P2 and 2 reported NEMO-deficient patients (NEMOX420W/Y and NEMOΔ4–10/Y). n = 3 or 4. (F) Impaired TNF-α production in P3-derived iPSC-Mϕ. Control and P3-derived iPSC-Mϕ were stimulated with LPS and IFN-γ for 4 hours before TNF-α determination by ELISA. TNF-α production of P3-derived iPSC-Mϕ was restored by reexpression of WT NEMO. Data are shown as the mean ± SD of 3 independent clones and are representative of 2 independent experiments. *P < 0.05, by unpaired, 2-tailed Student’s t test.