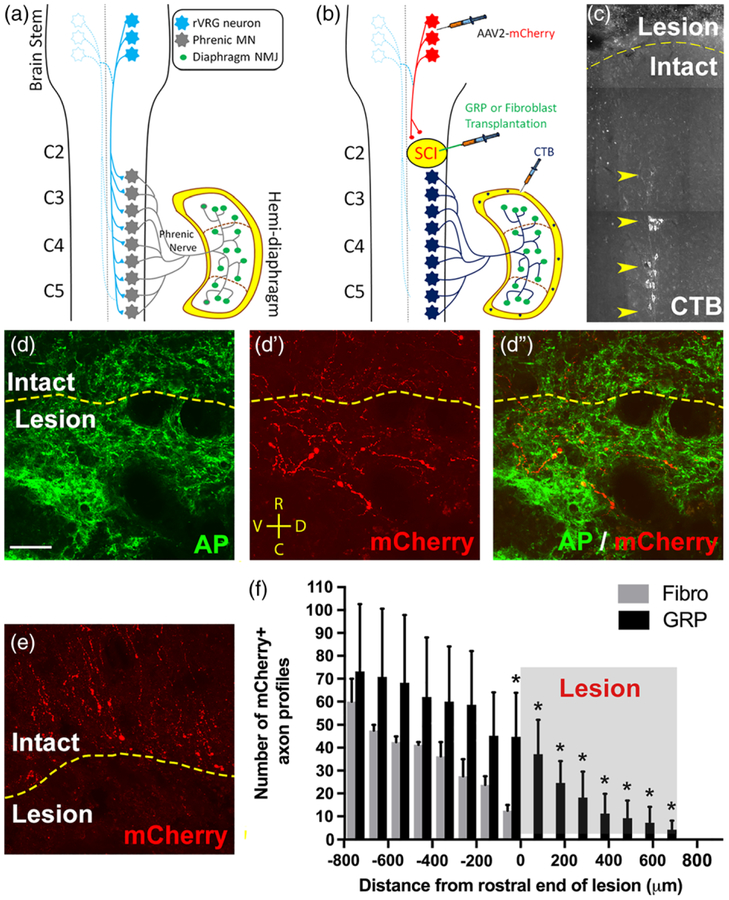
FIGURE 6.
GRPs stimulated robust regeneration of injured rVRG axons, but no synaptic reconnection of these regrowing fibers with PhMNs. Diagram illustrates the intact rVRG-PhMN-diaphragm circuit (a). To label rVRG axons in C2 hemisection rats receiving either GRP or fibroblast transplantation, we stereotaxically injected the anterograde axonal tracer AAV2-mCherry into the ipsilateral rVRG (b). We selectively labeled ipsilateral PhMNs by intrapleurally injecting the retrograde tracer cholera toxin B (b,c). GRPs stimulated robust regeneration of mCherry+ rVRG axons across the rostral intact-lesion border (d–d”). On the contrary, in the control fibroblast animals, all mCherry-labeled rVRG axons stop at the border between the rostral intact spinal cord and the lesion, with many of these axons retracting back from the injury site (e). No rVRG axons regenerate into the lesion site with fibroblast transplantation. Orientation of all images in panels d–d” and e: D = dorsal, V = ventral, R = rostral, C = caudal. Quantification of mCherry+ axon profiles at defined rostral-caudal distances relative to the rostral end of the lesion (f). GRPs reduced retraction of rVRG axons in the rostral intact spinal and stimulated significant regeneration across the entire lesion site (c). CTB labeling shows that the PhMN pool is located directly caudal to the hemisection (f). scale bar: 50 μm