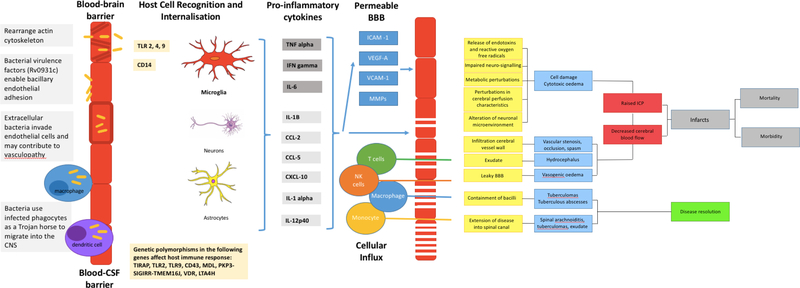
Figure 1: Summary of the pathogenesis of TBM.
A: Mycobacterium tuberculosis bacilli (M.tb) disseminate from the primary site of infection in the lung to seed the brain. The bacilli are able to traverse the blood brain barrier (BBB) and blood cerebrospinal fluid barrier (BCSFB) through various virulence factors that enable the invasion of and migration through cerebral vascular endothelial cells, or are carried into the central nervous system (CNS) by infected peripheral innate immune cells. B: In the CNS antigen recognition and internalisation by microglia, neurons and astrocytes occurs, mediated by numerous host genetic factors. C: The resulting immune response stimulates the release of pro-inflammatory cytokines and chemokines and other immune mediators that contribute to the breakdown of the BBB and the influx of innate and adaptive immune cells from the periphery. D: A prolific inflammatory response ensues. The inflammatory exudate in the basal cisterns contributes to cerebral vascular pathology and the development of hydrocephalus and raised intracranial pressure. Vasogenic oedema due to an influx of proteins through the leaky BBB, and cytotoxic oedema as a result of cellular damage contribute to the raised pressure. The overall decrease in cerebral blood flow puts the brain at risk of ischaemia, infarction and poor patient outcomes. In some cases the infection is controlled in discrete tuberculomas or abscesses, which resolve with treatment and time. Extension of the disease into the spinal canal manifests as spinal arachnoiditis, tuberculomas, or collection of exudate.