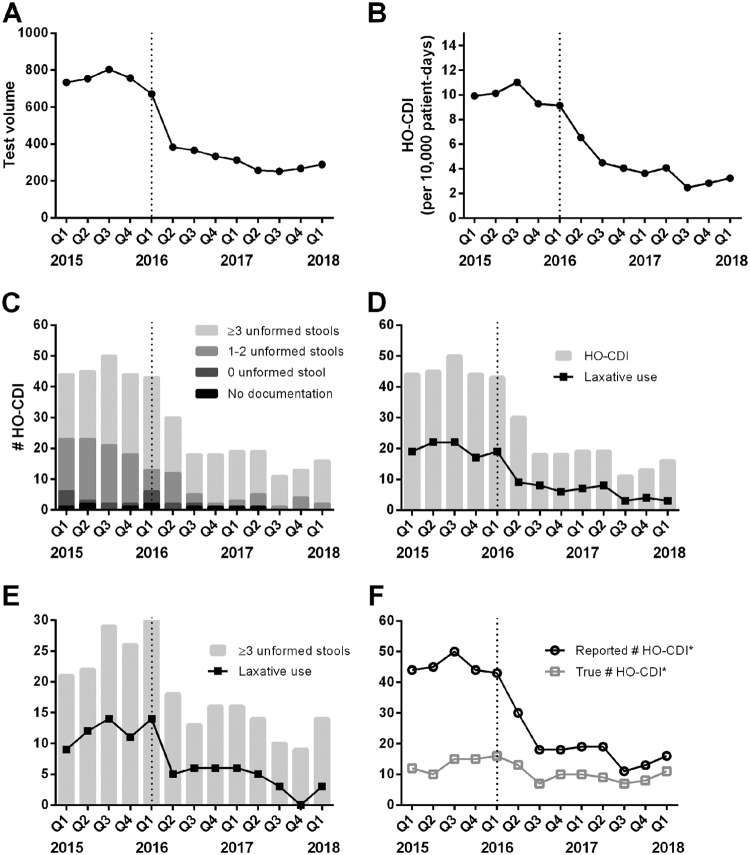
FIG 1.
Changes in Clostridioides difficile tests and CDI cases from January 2015 to March 2018, divided into quarters, with the dotted line indicating 2016 quarter 1 as the intervention period. (A) C. difficile test volumes for inpatients. (B) Reported HO-CDI rates. (C) Distribution of diarrheal episodes among LabID HO-CDI events. (D) Laxative use among LabID HO-CDI events. (E) Laxative use among LabID HO-CDI events with clinically significant diarrhea, defined as ≥3 unformed stools within 24 h prior to testing. (F) Numbers of reported HO-CDI events and true HO-CDI events, after removal of events with laxative use and/or insignificant diarrhea (<3 unformed stools within 24 h). The asterisks indicate the exclusion of 3 cases of non-HO-CDI events and 4 cases involving ileus or the use of a rectal tube throughout the study period.