FIG 1.
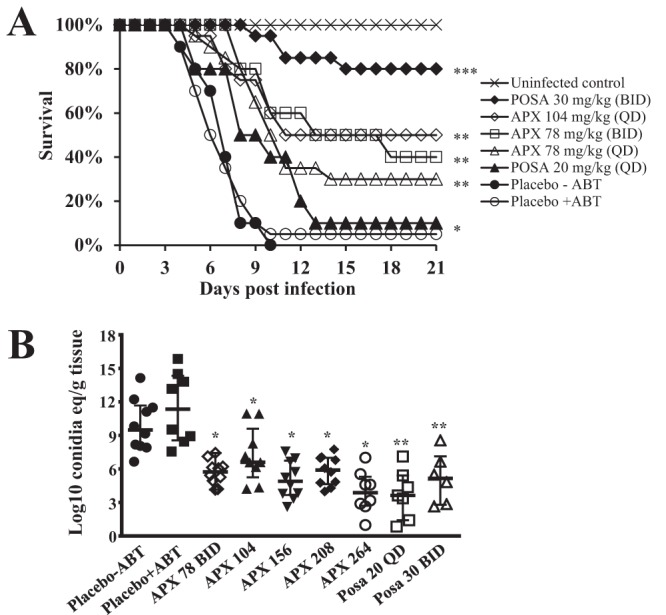
APX001 protects mice from IPA. (A) ICR male mice (n = 10 to 20) were infected with an average inoculum of 6.3 × 103 CFU per mouse via inhalation. Treatment was initiated 16 h after infection and continued daily for 7 days. A dose of 50 mg/kg ABT was administered orally 2 h prior to each APX001 dose. ***, P < 0.05 versus all other treatments; **, P < 0.0005 versus placebo control; *, P < 0.05 versus placebo control by log rank test. (B) Lung burdens in mice (6 to 10 per group) were measured at 4 days postinfection. Male ICR mice were infected with 6.7 × 103 CFU via inhalation. Treatment was initiated 16 h postinfection and continued for 4 days. APX001 was administered by oral gavage. ABT was administered orally 2 h prior to each APX001 dose. Mice were sacrificed 8 h after the last dose, and lungs were harvested and processed for tissue fungal burden by qPCR. Fungal burden data (presented as medians ± interquartile ranges) were log10 transformed and evaluated using the nonparametric Wilcoxon rank sum test (Prism 5; GraphPad Software, Inc., San Diego, CA). *, P < 0.009 versus placebo plus ABT; **, P < 0.004 versus placebo without ABT.
