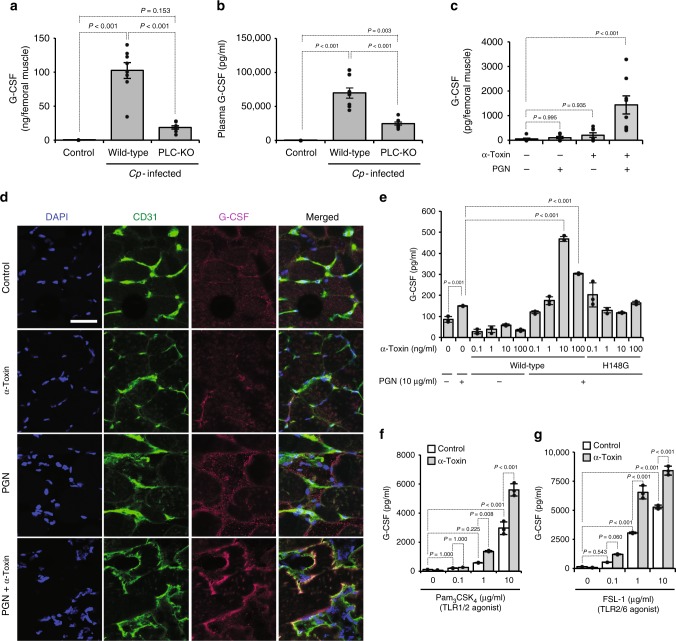
Fig. 1.
C. perfringens α-toxin accelerates the production of granulocyte colony-stimulating factor (G-CSF) in endothelial cells. a, b Mice were intramuscularly injected with 1 × 107 colony-forming units (CFUs) of C. perfringens Strain 13 (wild-type), phospholipase C knockout (PLC-KO), or TGY medium as a control (Control). At 24 h after infection, G-CSF levels in the infected muscle (a, n = 8 per condition) or plasma (b, n = 8 per condition) were measured by enzyme-linked immunosorbent assay. c, d Mice were injected intramuscularly with 20 ng of purified α-toxin and 100 μg of peptidoglycan (PGN). At 24 h after the administration, G-CSF levels in the muscle were determined (c, n = 8 per condition), or the muscle was subjected to immunohistochemical analysis with antibodies against CD31 and G-CSF (d). Scale bar, 40 µm. e Human umbilical vein endothelial cells (HUVECs) were cultured for 24 h in the presence or absence of the indicated concentrations of α-toxin (wild-type) or a variant α-toxin (H148G) and 10 μg ml−1 PGN. G-CSF levels in the culture medium were determined (n = 3 per condition). f, g HUVECs were cultured for 24 h in the presence or absence of 10 ng ml−1 α-toxin and the indicated concentration of Toll-like receptor 2 (TLR2) agonist, Pam3CSK4 (f, n = 3 per condition) or fibroblast-stimulating lipopeptide (FSL-1) (g, n = 3 per condition), and G-CSF levels in the culture medium were determined. One-way analysis of variance was employed to assess statistical significance. Values are mean ± standard error (a–c) or standard deviation (e–g)