Abstract
Plasma homocysteine level and megaloblastic anemia status are two factors that can affect the quality of life of patients with multiple sclerosis (MS). We conducted this study to determine the effect of vitamin B12 and folic acid supplementation on serum homocysteine, megaloblastic anemia status and quality of life of patients with MS. A total of 50 patients with relapsing remitting multiple sclerosis (RRMS) included in this study which divided into 2 groups. The vitamin group received 5 mg folic acid tablet daily and 3 doses of vitamin B12 (1,000 mcg) injection and the other group received placebo and normal saline injection (same doses). The quality of life was measured by using Multiple Sclerosis Quality of Life-54 questionnaire (MSQOL-54). Fully automated fluorescence polarization immunoassay was used to measure serum homocysteine, vitamin B12 and folate. Complete blood count blood test was conducted to determine the anemia status. The mean homocysteine level reduced by 2.49 ± 0.39 µmol/L (p = 0.001), hemoglobin increased from 11.24 ± 1.54 to 13.12 ± 1.05 g/dL (p = 0.001), and mean corpuscular volume decreased from 95.50 ± 6.65 to 89.64 ± 4.24 in the vitamin group (p = 0.001). There was a significant improvement in the mental field of life quality in the placebo group (37.46 ± 19.01 to 50.98 ± 21.64; p = 0.001), whereas both physical and mental fields of quality of life were improved significantly in the vitamin group (40.38 ± 15.07 to 59.21 ± 12.32 and 29.58 ± 15.99 to 51.68 ± 18.22, respectively; p = 0.001). Serum homocysteine level decrease and anemia status improvement with vitamin B12 and folic acid supplementation reveal the potential role of these two vitamins in improving the life quality of MS patients.
Trial Registration
Iranian Registry of Clinical Trials Identifier: IRCT2015100313678N7
Keywords: Multiple sclerosis, Homocysteine, Anemia, Vitamin B12, Folic acid, Quality of life
INTRODUCTION
Multiple sclerosis (MS) is a relatively common chronic neurological disorder in which demyelination of axons of neurons occurs in different areas of the central nervous system [1]. Currently, more than 1.3 million people are living with this disease all over the globe [2]. According to World Health Organization records, the prevalence of MS in Iran is 20–60 persons per 100,000 of the population [3]. The age of onset is typically between 20–40 years old but it could rarely occur even at the age of 2 or may occur in the eighth decade of life [4]. The risk of the disease is 3–4 times more in females compared to males. [5].
Relapsing remitting multiple sclerosis (RRMS) is defined as MS in which patients have relapses of MS and periods of stability in between relapses. Relapses are episodes of new or worsening symptom not caused by fever or infection and that last more than 48 hours. In other words, a stable course is punctuated by episodes of new or worse symptoms. RRMS is the most common initial form of MS. Younger patients are more likely to have this form of MS than older patients [6].
Patients with chronic diseases such as MS face problems related to their illness. These problems limit the health-related activities of the patients and have a negative impact on their quality of life [7]. The quality of life has several dimensions and aspects that includes physical and psychological health which has received much attention recently due to information technology advancements in the field of health policies [8]. In recent years, studies have examined the factors associated with the quality of life of MS patients. Many symptoms associated with MS can greatly affect the quality of life of these patients [9].
Recent studies have focused on the role of vitamin B12, folate, and homocysteine in MS. Increased levels of homocysteine have detrimental effects on the human nervous system [10]. Serum homocysteine levels of MS patients are higher than of the healthy individuals as one of the causative factors in the pathogenesis of MS [10,11,12,13]. Also, lack of vitamin B12 will lead to methionine and S-adenosylmethionine deficiencies while both are required for the myelin synthesis. Inhibition of S-adenosylmethionine synthesis due to vitamin B12 deficiency causes some demyelination lesions in pernicious anemia which is a type of megaloblastic anemia [14]. There are remarkable similarities in the epidemiology of MS and pernicious anemia. Having said that, recent investigations have shown increased risk of macrocytosis and megaloblastic anemia in patients with MS [15].
There is a possible link between the onset of the first neurological signs of MS and serum levels of vitamin B12 and folic acid of the patient at the time [4]. These findings indicate that lack of vitamin B12 and folic acid and MS, both have similar symptoms. In addition, increased intake of vitamin B12 and folate with immunotherapy treatments has shown promising results in MS patients [16]. A meta-analysis study in 2011 was conducted on MS patients to find a relationship between vitamin B12 and other micronutrients. They found a direct relationship between low vitamin B12 levels and the risk of MS [17].
Most of the recent studies have reported increased levels of plasma homocysteine in MS patients; however, a few case-control studies did not find a significant difference in plasma homocysteine levels between their case and control groups and which could be possibly due to patients' specific diets like Mediterranean diet [17].
If only folate supplementation was applied for patients with cobalamin deficiency, it might improve anemia status, but it could not stop the neurological disorders, and if only cobalamin supplementation was used for patients with folate deficiency, it might not treat their anemia [18].
According to above, we decided to conduct this study to determine the effect of vitamin B12 and folic acid supplementation on serum homocysteine, anemia status and quality of life of RRMS patients. As mentioned above, this type of disease is the most common type of MS, which is the reason why we have chosen it for this study.
MATERIALS AND METHODS
Study population
Prior to the start of our study, it was approved by the ethics committee of the Urmia University of Medical Sciences and the International Centre of Iranian Registry of Clinical Trial (registration number: IRCT2015100313678N7). Written informed consent which included complete information about the study and dietary supplements and also confidentiality of their private information was obtained from all participants.
This double-blinded clinical trial was performed in Farabi Hospital, Kermanshah (Iran) on 2 groups of RRMS patients—the vitamin group and the placebo group. The diagnosis of RRMS was based on the revised version of MS diagnostic method (McDonald Criteria) [19]. Inclusion criteria included non-smokers patients diagnosed with RRMS aged between 20 to 40 years. None of the patients had received vitamin B12 and folate supplementary products, corticosteroids, betaine, and choline supplements in the past 6 months. Additionally, patients with any history of other neurological diseases or severe depression, renal failure, traumatic brain injury, and cardiovascular disease were all excluded.
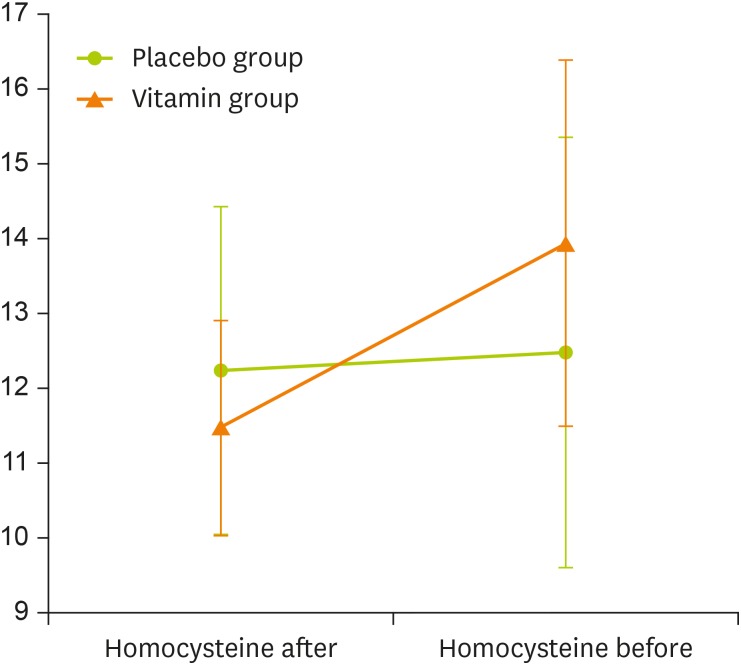
Two trained nurses obtained blood samples from the subjects and also guided them to complete the questionnaires. The vitamin group included 25 RRMS patients that took 5 mg daily folic acid tablets and three divided doses of 1mg injective vitamin B12. Immediately after completing the questionnaire, blood sample were taken for biochemical measurements including complete blood count (CBC) blood test and serum homocysteine level and blood pressure were measured, and the first dose of vitamin B12 was injected. The patients in the vitamin group then received their second dose a month after the first visit, and finally, the third dose was injected a month after the previous injection. The placebo group included 25 RRMS patients who had no significant difference in age and sex distributions compared to the vitamin group. The placebo group of patients received placebo in similar amounts of vitamin group (color- and size-matched starch made tablets and normal saline administration). The patients completed the validated Persian version of Multiple Sclerosis Quality of Life-54 questionnaire (MSQOL-54). The questionnaire included 14 aspects of quality of life such as physical health, and role limitations due to physical problems. Scores ranged from 0 to 100 for each field, higher scores indicated better conditions [20]. The intervention was carried out for 2 months, and at the end of the intervention, 7 days after the last injection of vitamin B12 (an appropriate time to determine the effect of these supplements in the body), CBC blood test, serum homocysteine level, and the blood pressure of 2 groups were measured. Finally, the MSQOL-54 was completed by the patients again. All patients were examined at the end of the intervention, and none of the subjects left the study (Figure 1).
Figure 1. Repeated measure of homocysteine changes in 2 groups (adjusted for baseline); group code 0: placebo group and group code 1: vitamin group.
Laboratory measurements
In order to measure total serum homocysteine, hemoglobin, mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC), blood samples (5 mL) were obtained from the antecubital vein of each subject. Measurement of hemoglobin, MCV and MCHC was accomplished through the CBC test using Cell Counter Sysmex XP-300 model (Sysmex, Kobe, Japan). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the subjects were measured by mercury sphygmomanometer (ALPK2-300V; Tanaka Sangyo Co., Ltd., Tokyo, Japan) and have the same corrections in the same ones.
Total serum folate, vitamin B12 and homocysteine were easured with chemiluminescence microparticle immunoassay/Abbott biochemical method using Abbott IMX kits with Abbott IMx® unit (Abbott Diagnostics, Lake Forest, IL, USA). Blood samples were kept on ice until serum separated.
The Abbott Homocysteine assay has been introduced as the primary method of measuring total serum homocysteine by the National Health and Nutrition Examination. Abbott IMX homocysteine assay is a fully automated fluorescence polarization immunoassay (FPIA). Dithiothreitol reduces homocysteine bound to albumin and some other small molecules, homocysteine, mixed disulfide and free thiol compositions. S-adenosyl-homocysteine (SAH) hydrolase converts homocysteine to SAH in the presence of adenosine was added to the test environment. In the next step, the specific monoclonal antibody with the fluoresceinated SAH analog tracer makes up the FPIA diagnostic system. This method has been chosen by this organization as a reference assay in small and medium-scale studies [21].
Statistics
The data were expressed as mean ± standard deviation (SD). Kolmogorov-Smirnov test was used in order to test the normality of data. The independent and dependent t-tests have been performed for the comparison of parametric data, and in order to compare non-parametric data, we applied Mann-Whitney and Wilcoxon tests. The χ2 test was used to determine the distribution of serum homocysteine levels in the patients. All graphs were plotted with the R software (v 3.4.1; R Project for Statistical Computing, Vienna, Austria). The analysis was performed by the 23rd version of SPSS software (SPSS Inc., Chicago, IL, USA). We considered p < 0.05 to be statistically significant.
RESULTS
There were no significant differences in terms of age, sex, marital status, serum vitamin B12 and folate distribution between two groups. However, the number of single patients in both groups was higher than the number of married ones (p > 0.05) (Table 1).
Table 1. General variables of studied population.
| Variables | Groups | p value | ||
|---|---|---|---|---|
| Vitamin (n = 25) | Placebo (n = 25) | |||
| Age | 30.16 ± 4.99 | 30.52 ± 4.97 | 0.799 | |
| Sex | 0.782 | |||
| Male | 12 (48) | 11 (44) | ||
| Female | 13 (52) | 14 (56) | ||
| Marriage | 0.780 | |||
| Single | 14 (56) | 15 (60) | ||
| Married | 11 (44) | 10 (40) | ||
| Folate (ng/mL) | 5.08 ± 2.01 | 5.33 ± 1.36 | 0.648 | |
| Vitamin B12 (pg/mL) | 221.16 ± 72.65 | 257.33 ± 104.57 | 0.187 | |
The data were expressed as mean ± standard deviation or number of patients (%).
The analysis of data has shown that MCV, SBP and DBP had a non-normal distribution. However, the other variables were normally distributed. The examination of the variables showed that there was no significant difference between all variables in two groups at the baseline (Table 2). As shown in Table 2, unlike other variables, there was a significant change in the mental health composite score of the placebo group after the intervention (p < 0.05). On the other hand, significant differences have arisen in homocysteine, hemoglobin, MCV, and both fields of quality of life in vitamin group (p < 0.05); while there was no significant change in MCHC, SBP and DBP of the vitamin group (p > 0.05).
Table 2. Studied variables in vitamin and placebo groups, before and after the intervention.
| Variables | Placebo | Vitamin | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | After intervention | Baseline | After intervention | p∥ | p¶ | p** | p†† | |
| Homocysteine (µmol/L) | 12.48 ± 2.88 | 12.23 ± 2.19 | 13.94 ± 2.45 | 11.45 ± 1.44 | 0.059* | 0.294† | 0.001† | 0.146* |
| Hemoglobin (g/dL) | 11.16 ± 1.67 | 11.12 ± 0.95 | 11.24 ± 1.54 | 13.12 ± 1.05 | 0.861* | 0.814† | 0.001† | 0.001* |
| MCV (pg/cell) | 95.76 ± 6.90 | 94.47 ± 4.62 | 95.50 ± 6.65 | 89.64 ± 4.24 | 0.734‡ | 0.187§ | 0.001§ | 0.001‡ |
| MCHC (g/dL) | 31.68 ± 2.46 | 31.73 ± 1.24 | 31.83 ± 2.05 | 31.75 ± 0.91 | 0.814* | 0.910† | 0.807† | 0.959* |
| Systolic BP (mmHg) | 112.00 ± 12.42 | 110.60 ± 12.52 | 111.80 ± 11.35 | 110.00 ± 9.01 | 0.961‡ | 0.142§ | 0.219§ | 0.819‡ |
| Diastolic BP (mmHg) | 74.20 ± 4.49 | 75.40 ± 4.06 | 74.40 ± 4.40 | 74.00 ± 3.82 | 0.859‡ | 0.360§ | 0.685§ | 0.278‡ |
| PCS | 44.12 ± 21.68 | 42.70 ± 22.37 | 40.38 ± 15.07 | 59.21 ± 12.32 | 0.482* | 0.249† | 0.001† | 0.314* |
| MCS | 37.46 ± 19.01 | 50.98 ± 21.64 | 29.58 ± 15.99 | 51.68 ± 18.22 | 0.720* | 0.001† | 0.001† | 0.001* |
Data are shown as mean ± standard deviation.
MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; PCS, Physical Health Composite Score; MCS, Mental Health Composite Score.
*Independent t-test; †paired t-test; ‡Mann-Whitney test; §Wilcoxon test. The p value comparison of the measured; ∥variables in the vitamin and placebo groups at the baseline, ¶variables in the placebo group before and after the intervention, **variables in the vitamin group before and after the intervention, and ††variables in the vitamin and placebo groups after the intervention.
Due to the difference (but non-significant) between the mean of serum homocysteine of 2 groups at the baseline (lower level of the mean homocysteine in the placebo group compared to vitamin group; p = 0.059), the arisen difference in homocysteine level after the intervention cannot be shown only by comparing the means (Figure 1). In order to overcome this situation, the statistical analysis of the mean difference of homocysteine levels of 2 groups and also other variables was conducted (Table 3). The results showed a significant difference in the mean homocysteine of 2 groups before and after the intervention (p = 0.001). Also, the mean difference of mental health composite score was statistically significant.
Table 3. Mean differences of studied variables of 2 groups, before and after the intervention.
| Variable | Values | p value |
|---|---|---|
| Homocysteine (µmol/L) | 2.24 ± 0.33 | 0.001* |
| Hemoglobin (g/dL) | −1.92 ± 0.19 | 0.001† |
| MCV (pg/cell) | 4.57 ± 1.17 | 0.001* |
| MCHC (g/dL) | 0.13 ± 0.55 | 0.806* |
| SBP (mmHg) | 0.40 ± 1.90 | 0.992† |
| DBP (mmHg) | 1.60 ± 1.53 | 0.229† |
| PCS | −20.52 ± 7.70 | 0.004* |
| MCS | −8.57 ± 3.46 | 0.029† |
Data are shown as mean differences ± standard deviation.
MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; SBP, systolic blood pressure; DBP, diastolic blood pressure; PCS, Physical Health Composite Score; MCS, Mental Health Composite Score.
*Independent t-test; †Mann-Whitney test.
Furthermore, χ2 test has been used to evaluate the distribution of homocysteine level (Table 4). As shown in Table 4, all the patients in vitamin group that had serum homocysteine levels higher than 13.9 µmol/L, reached the normal range (< 13.9 µmol/L) after the treatment (p = 0.001). However, in the placebo group, results showed that 9 patients had high levels of homocysteine at the baseline. The number of patients with a high level of homocysteine was six after the intervention, but the results of analysis demonstrated that this change was not significant (p = 0.335) (Table 4).
Table 4. Distribution of normal and abnormal homocysteine levels in vitamin and placebo groups, before and after the intervention.
| Groups | Less than 13.9 µmol/L (person) | More than 13.9 µmol/L (person) | Total | χ2 | p value | df | |
|---|---|---|---|---|---|---|---|
| Vitamin group | 19.44 | 0.001 | 1 | ||||
| Pre-intervention | 11 | 14 | 25 | ||||
| Post-intervention | 25 | 0 | 25 | ||||
| Placebo group | 0.857 | 0.335 | 1 | ||||
| Pre-intervention | 16 | 9 | 25 | ||||
| Post-intervention | 19 | 6 | 25 | ||||
The χ2 test has been used to evaluate the distribution of homocysteine level.
DISCUSSION
The effect of debilitating chronic diseases such as MS and their attendant problems, including the impact of disability on quality of life in all dimensions is obvious [22]. Increasing the consumption of folic acid and vitamin B12 improved physical and mental dimensions of quality of life which is in line with the findings of our study [23]. Zhang et al. [24] have shown that vitamin B12 can reduce pain in some neurological diseases such as MS. This effect was also seen in the vitamin group of the present study. Pietro et al. [23] studied on 2 groups of patients with MS and reported that the group of subjects who consumed more than 4.2 µg of vitamin B12 on daily bases had a greater improvement in the quality of life compared to the group that consumed less than 4.2 µg of this vitamin per day. The results of the present study indicated that vitamin B12 and folic acid consumption significantly improved both physical and mental dimensions of life quality in the vitamin group; but in the placebo group, improvements were only limited to the psychological dimension of quality of life and no significant change in the physical dimension was observed.
Sivakumar et al. [25] suggested that decreasing plasma levels of vitamin B12 can reduce physical performance; however, this effect can be lifted by consuming vitamin B12 rich-foods by the patients. A study was conducted by Ramsaransing et al. [13] in 2006 concluded that the increased level of serum homocysteine in patients with MS was not associated with disease progression. While, unlike their findings, we found an increase of serum homocysteine in all types of MS. Their study showed that levels of vitamin B12 in MS patients and healthy people did not differ significantly, which conflicts with the findings of the present study. However, the results of research by Kira et al. [26] showed a significant decrease in the binding capacity of unsaturated serum vitamin B12 in MS patients compared to healthy subjects. In the study of Ramsaransing et al. [13], the mean homocysteine level in patients with RRMS type was meaningfully higher compared to their control group. In Moghaddasi et al.'s study [12], the mean serum level of homocysteine in MS patients also was elevated significantly compared with healthy controls.
Baig et al. [27] showed that the elevated serum homocysteine levels in MS patients are associated with a significant decrease in serum and cerebrospinal fluid levels of vitamin B12 which confirms the findings of the present research. Besler and Comoğlu [28] showed that the reduced levels of folate and vitamin B12 in the patients are associated with increased homocysteine levels, but the comparison between their case and control groups was not significant. However, Moghaddasi et al. [12] observed a significant association between decreasing folate and vitamin B12 levels and increasing levels of serum homocysteine. In a study of patients with hypercholesterolemia, conducted by Shidfar et al. [29], mean serum homocysteine dropped significantly by taking folate supplements, whereas the mean homocysteine levels were not significantly different in their placebo group.
The need for vitamin B12 seems to be increased in chronic autoimmune diseases (e.g. MS). Therefore, we can expect increased levels of homocysteine in these types of diseases due to the reduced levels of vitamin B12 and folate which leads to changes in the myelin stability [13].
Previous studies have reported lower levels of vitamin B12 in MS patients [17]. They have posed a possible disorder in the metabolism of this vitamin in MS. Also, the reduced serum levels of vitamin B12 in patients with MS that treated with interferon H and Copaxone are considered and some studies have extended the role of these drugs in an increased need for this vitamin in order to myelin repair [16,30].
Increased levels of serum R-binder protein and a reduction in transcobalamin II in some MS patients in a recent study have been shown [31]. Also, transcobalamin II deficiency leads to megaloblastic anemia. In 1959 Plum and Fog [32] showed that the average size of red blood cells (MCV) in MS patients increases and there is a possible link between MS and megaloblastic anemia. Crellin et al. [33] showed the mean of MCV in MS patients was significantly higher compared to healthy individuals. Also in 1991, a study was conducted to evaluate the macrocytosis status in patients with MS. The results indicated that MS patients had significant macrocytosis in comparison with healthy subjects [34]. Al-Din et al. [34] also measured hemoglobin level in their subjects and the results showed that hemoglobin levels in MS patients were significantly higher than healthy controls.
The possible cause of low hemoglobin levels in our subjects can be explained by referring to study of Deleva [35] in which the researchers examined the relationship between MS and different types of anemia (anaemic syndrome). In their study, 18 MS patients (13 of them were diagnosed with the relapsing-remitting type of MS) were examined in terms of some types of anemia. They described the subjects as eight patients with pernicious anemia, three patients with iron deficiency anemia, 6 patients with vitamin B12 deficiency, and one patient who was diagnosed with heterozygous thalassemia. Deleva [35] concluded that there is a significant relationship between MS and various types of anemia. Therefore, the possible cause of low hemoglobin level in most patients of the present study could be due to anaemic syndrome and multifactorial anemia.
van Dijk et al. [36] studied the variations of blood pressure in patients who were treated with folic acid in order to reduce their homocysteine levels. During 2 years of intervention, the patients mean systolic and diastolic blood pressure decreased significantly. In our study, there was no significant change in the blood pressure after supplementation with folate and vitamin B12. A potential cause of steady blood pressure after taking folic acid in this study was the short duration of the intervention. Also, due to many factors affecting blood pressure, controlling these confounding factors need more complex research in longer durations.
CONCLUSION
Current dietary recommendations for patients with MS and other chronic diseases can be very simple for these complex situations. Results of the present study have shown that homocysteine levels, anemia status, and eventually quality of life of patients with MS can be significantly improved by administration 1mg of vitamin B12 monthly and adding rich-food sources of folic acid on their diet. To answer the question of why MS patients must consume more of these vitamins; we have to investigate the nature of this disease that is associated with megaloblastic and pernicious anemia and recent studies have clearly shown this correlation. Recent therapies cannot truly treat the disease or reverse its progression. Thus, some replacements for these therapies are required. Further studies in the field of MS dietary patterns must be conducted. Based on the potential role of vitamin B12 and folic acid in the improvement of physical and mental dimensions of life quality, increasing consumption of foods that provide adequate amounts of these 2 vitamins and appropriate dietary interventions for these patients are recommended.
ACKNOWLEDGEMENTS
This study was conducted under the supervision of Urmia University of Medical Sciences. We would like to thank those who participated and helped us in conducting this study, including all the patients, Mrs. Javidan and the Head of Kermanshah MS Society.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Winquist RJ, Kwong A, Ramachandran R, Jain J. The complex etiology of multiple sclerosis. Biochem Pharmacol. 2007;74:1321–1329. doi: 10.1016/j.bcp.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Kingwell E, Bajdik C, Phillips N, Zhu F, Oger J, Hashimoto S, Tremlett H. Cancer risk in multiple sclerosis: findings from British Columbia, Canada. Brain. 2012;135:2973–2979. doi: 10.1093/brain/aws148. [DOI] [PubMed] [Google Scholar]
- 3.Etemadifar M, Sajjadi S, Nasr Z, Firoozeei TS, Abtahi SH, Akbari M, Fereidan-Esfahani M. Epidemiology of multiple sclerosis in Iran: a systematic review. Eur Neurol. 2013;70:356–363. doi: 10.1159/000355140. [DOI] [PubMed] [Google Scholar]
- 4.Sandyk R, Awerbuch GI. Vitamin B12 and its relationship to age of onset of multiple sclerosis. Int J Neurosci. 1993;71:93–99. doi: 10.3109/00207459309000596. [DOI] [PubMed] [Google Scholar]
- 5.Idiman E, Uzunel F, Ozakbas S, Yozbatiran N, Oguz M, Callioglu B, Gokce N, Bahar Z. Cross-cultural adaptation and validation of multiple sclerosis quality of life questionnaire (MSQOL-54) in a Turkish multiple sclerosis sample. J Neurol Sci. 2006;240:77–80. doi: 10.1016/j.jns.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Holmøy T, Torkildsen Ø, Myhr KM. An update on cladribine for relapsing-remitting multiple sclerosis. Expert Opin Pharmacother. 2017;18:1627–1635. doi: 10.1080/14656566.2017.1372747. [DOI] [PubMed] [Google Scholar]
- 7.Morgante L. Hope in multiple sclerosis: a nursing perspective. Int J MS Care. 2000;2:9–15. [Google Scholar]
- 8.Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187–206. doi: 10.1007/BF02260859. [DOI] [PubMed] [Google Scholar]
- 9.Miller DM, Rudick RA, Baier M, Cutter G, Doughtery DS, Weinstock-Guttman B, Mass MK, Fisher E, Simonian N. Factors that predict health-related quality of life in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2003;9:1–5. doi: 10.1191/1352458503ms888oa. [DOI] [PubMed] [Google Scholar]
- 10.Ansari R, Mahta A, Mallack E, Luo JJ. Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol. 2014;10:281–288. doi: 10.3988/jcn.2014.10.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashtari F, Abari SS, ShayganNejad V. Serum homocysteine level in patients with multiple sclerosis. J Res Med Sci. 2005;10:302–304. [Google Scholar]
- 12.Moghaddasi M, Mamarabadi M, Mohebi N, Razjouyan H, Aghaei M. Homocysteine, vitamin B12 and folate levels in Iranian patients with multiple sclerosis: a case control study. Clin Neurol Neurosurg. 2013;115:1802–1805. doi: 10.1016/j.clineuro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Ramsaransing GS, Fokkema MR, Teelken A, Arutjunyan AV, Koch M, De Keyser J. Plasma homocysteine levels in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77:189–192. doi: 10.1136/jnnp.2005.072199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds EH. Multiple sclerosis and vitamin B12 metabolism. J Neurol Neurosurg Psychiatry. 1992;55:339–340. doi: 10.1136/jnnp.55.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis. J Clin Neurosci. 2009;16:399–403. doi: 10.1016/j.jocn.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Gupta JK, Ingegno AP, Cook AW, Pertschuk LP. Multiple sclerosis and malabsorption. Am J Gastroenterol. 1977;68:560–565. [PubMed] [Google Scholar]
- 17.Zhu Y, He ZY, Liu HN. Meta-analysis of the relationship between homocysteine, vitamin B12, folate, and multiple sclerosis. J Clin Neurosci. 2011;18:933–938. doi: 10.1016/j.jocn.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M, Blackmore S. Chapter 8. Investigation of megaloblastic anaemia-cobalamin, folate, and metabolite status. In: Lewis SM, Bain BJ, Bates I, Dacie JV, editors. Dacie and Lewis Practical Haematology. 10th ed. Philadelphia (PA): Churchill Livingstone Elsevier; 2006. pp. 161–185. [Google Scholar]
- 19.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O'Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 20.Ghaem H, Borhani Haghighi A, Jafari P, Nikseresht AR. Validity and reliability of the Persian version of the multiple sclerosis quality of life questionnaire. Neurol India. 2007;55:369–375. doi: 10.4103/0028-3886.33316. [DOI] [PubMed] [Google Scholar]
- 21.Sampson EJ. Laboratory procedure manual: total homocysteine in plasma by Abbott IMX [Internet] [cited 2017 March 21]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2001-2002/labmethods/l06_b_met_homocysteine_imx.pdf.
- 22.Madani H, Navipoor H, Roozbayani P. Effect of self-care program on the self-esteem of multiple sclerosis patients. ZUMS J. 2002;10:47–50. [Google Scholar]
- 23.Pietro KJ, Jensen AM, Schumacher JR, Anderson JW. Vitamin B12 intake correlated to physical and mental improvements in multiple sclerosis specific quality of life. Int J Adv Nutr Health Sci. 2014;2:98–108. [Google Scholar]
- 24.Zhang M, Han W, Hu S, Xu H. Methylcobalamin: a potential vitamin of pain killer. Neural Plast. 2013;2013:424651. doi: 10.1155/2013/424651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivakumar B, Nair KM, Sreeramulu D, Suryanarayana P, Ravinder P, Shatrugna V, Kumar PA, Raghunath M, Rao VV, Balakrishna N, Kumar PU, Raghuramulu N. Effect of micronutrient supplement on health and nutritional status of schoolchildren: biochemical status. Nutrition. 2006;22:S15–25. doi: 10.1016/j.nut.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Kira J, Tobimatsu S, Goto I. Vitamin B12 metabolism and massive-dose methyl vitamin B12 therapy in Japanese patients with multiple sclerosis. Intern Med. 1994;33:82–86. doi: 10.2169/internalmedicine.33.82. [DOI] [PubMed] [Google Scholar]
- 27.Baig SM, Ali Qureshi G. Homocysteine and vitamin B12 in multiple sclerosis. Biog Amines. 1995;11:479–485. [Google Scholar]
- 28.Besler HT, Comoğlu S. Lipoprotein oxidation, plasma total antioxidant capacity and homocysteine level in patients with multiple sclerosis. Nutr Neurosci. 2003;6:189–196. doi: 10.1080/1028415031000115945. [DOI] [PubMed] [Google Scholar]
- 29.Shidfar F, Homayounfar R, Fereshtehnejad SM, Kalani A. Effect of folate supplementation on serum homocysteine and plasma total antioxidant capacity in hypercholesterolemic adults under lovastatin treatment: a double-blind randomized controlled clinical trial. Arch Med Res. 2009;40:380–386. doi: 10.1016/j.arcmed.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Kargiotis O, Paschali A, Messinis L, Papathanasopoulos P. Quality of life in multiple sclerosis: effects of current treatment options. Int Rev Psychiatry. 2010;22:67–82. doi: 10.3109/09540261003589521. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds EH, Bottiglieri T, Laundy M, Crellin RF, Kirker SG. Vitamin B12 metabolism in multiple sclerosis. Arch Neurol. 1992;49:649–652. doi: 10.1001/archneur.1992.00530300089014. [DOI] [PubMed] [Google Scholar]
- 32.Plum CM. Studies in multiple sclerosis. I. Acta Psychiatr Neurol Scand Suppl. 1959;128:1–94. [PubMed] [Google Scholar]
- 33.Crellin RF, Bottiglieri T, Reynolds EH. Multiple sclerosis and macrocytosis. Acta Neurol Scand. 1990;81:388–391. doi: 10.1111/j.1600-0404.1990.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 34.Najim al-Din AS, Khojali M, Habbosh H, Farah S, Idris AR, al-Muhtasib F. Macrocytosis in multiple sclerosis. A study in 82 de novo Arab patients. J Neurol Neurosurg Psychiatry. 1991;54:415–416. doi: 10.1136/jnnp.54.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deleva NS. Multiple sclerosis associated with anaemic syndrome: a retrospective analysis and literature review. J IMAB. 2012;18:203–205. [Google Scholar]
- 36.van Dijk RA, Rauwerda JA, Steyn M, Twisk JW, Stehouwer CD. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: a 2-year, randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2001;21:2072–2079. doi: 10.1161/hq1201.100223. [DOI] [PubMed] [Google Scholar]