Fig. 6.
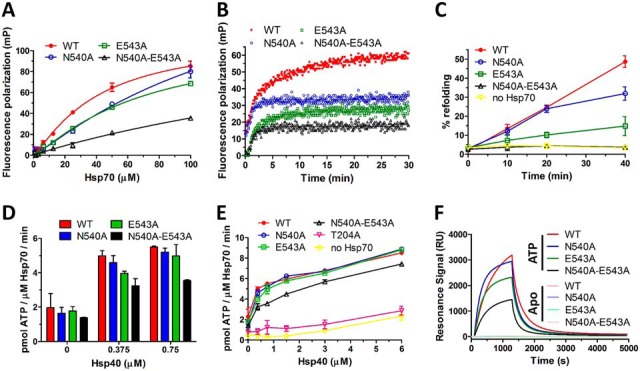
E543A and N540A-E543A mutants have severely impaired substrate binding/refolding activities and lower interaction with Hsp40. A, Equilibrium binding curves of F-NRLLLTG peptide binding to Hsp70 WT and mutants under nucleotide-free conditions. Fluorescence polarization was determined at 30 nm peptide and increasing Hsp70 concentrations. Error bars represent S.D.; n = 3 independent experiments. B, Kinetics of F-NRLLLTG interaction with WT and mutant Hsp70s under nucleotide- free conditions determined by fluorescence polarization. Protein and peptide concentrations were 50 μm and 30 nm, respectively. C, Firefly luciferase was chemically denatured, mixed with Hsp70 WT or mutants (1 μm), Hsp40 (2 μm), Bag-1 (0.5 μm) and ATP (2 mm) and recovered luminescence was measured. Error bars represent S.D.; n = 3 independent experiments. D, ATPase activity of Hsp70 WT and mutants at lower Hsp40 concentrations, for full results see E. E, ATPase activity of Hsp70 WT and mutants (2 μm) was tested at various Hsp40 concentrations in malachite green assay. Error bars represent S.D.; n = 3 independent experiments. F, SPR measurement of Hsp70 WT and mutants binding to Hsp40 under nucleotide-free conditions (Apo) or in the presence of ATP.
