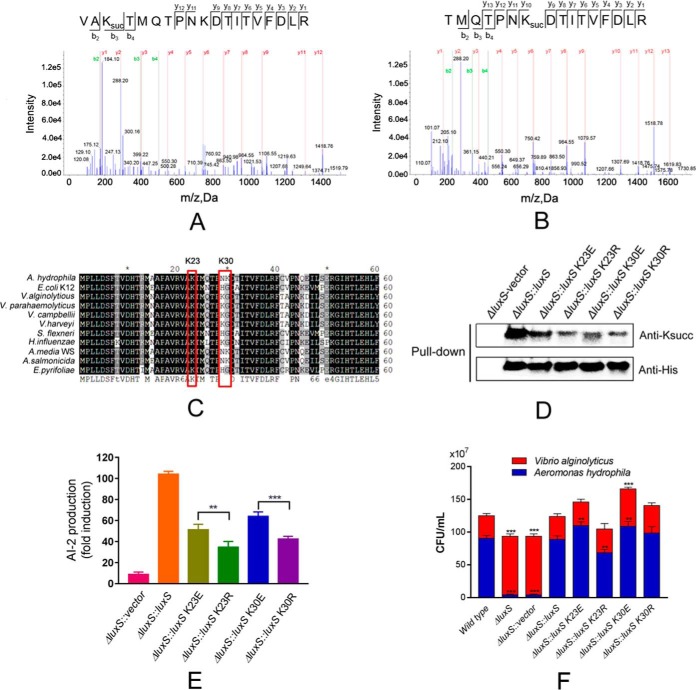
Fig. 6.
Lysine-succinylation of LuxS affects the quorum sensing properties of A. hydrophila. A and B, MS/MS spectral identification of succinyl-peptides on the K23 and K30 sites of A. hydrophila LuxS, respectively; C, Alignment of partial LuxS sequence (1-60 aa) homologs from ten bacterial species, including E. coli, V. alginolytious (WP_017820391.1), V. parahemeolyticus (WP_025792841.1), V. campbellii (WP_012128886.1), V. harveyi (WP_042603130.1), and Shigella flexneri. Conserved sites are shaded in gray/black and residues of interest are highlighted; D, Acetylation levels of purified LuxS and its derivatives were determined by Western blotting using anti-acetyllysine and anti-His tag antibodies, respectively. ΔluxS with the original vector was used as the negative control; E, AI-2 activity of ΔluxS complement strains and derivatives, as determined by V. harveyi BBl70 bioluminescence assays; F, Effect of site-directed mutagenesis of LuxS on bacterial competition between A. hydrophila and Vibrio alginolyticus. Statistical analysis was performed with t tests of different groups. p values < 0.0001 are indicated by *** and p values < 0.05 are indicated by **; Wild type A. hydrophila as the positive control with ΔluxS, and ΔluxS containing the original vector as the negative control; Red color indicates Vibrio alginolyticus, whereas blue indicates A. hydrophila.