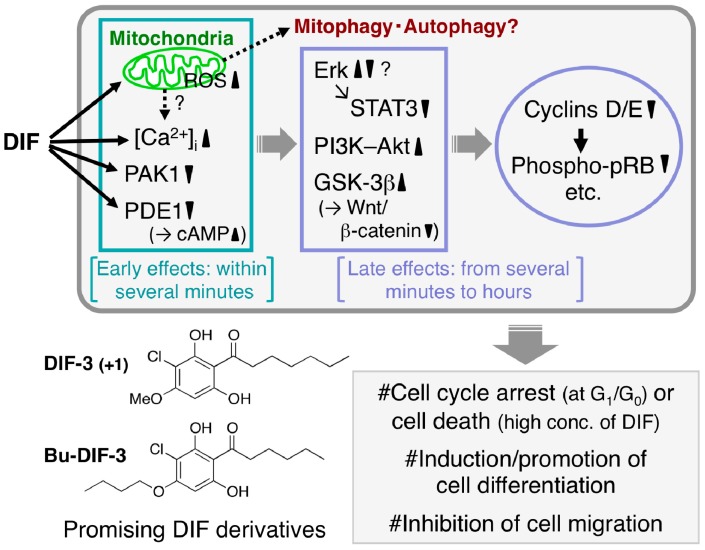
Figure 4.
Proposed scheme of the antitumor effects of DIFs. After the addition of one of the DIFs to tumor cells, the DIF rapidly (within several minutes) disturbs mitochondrial function [69,77], increases reactive oxygen species (ROS) production [69] and intracellular calcium concentration ([Ca2+]i) [63,64,69,77] and inhibits the activities of p21-activated kinase 1 (PAK1) [74] and calmodulin-dependent cAMP/cGMP phosphodiesterase (PDE1) (resulting in an increase in cAMP levels) [76]. Over time (from several minutes to hours) the DIF also affects the activities of extracellular signal-regulated kinase (Erk), signal transducer and activator of transcription 3 (STAT3), phosphatidylinositol 3-kinase (PI3K)–Akt, glycogen synthase kinase-3β (GSK-3β) and the Wnt/β-catenin pathway, which suppresses the expression of cyclins D/E (and promotes the degradation of cyclin D1) and the subsequent reduction of phospho-pRB [65,66,74,77,78,79,80,81]. At appropriate concentrations the DIFs have been found to induce growth arrest in all the tumor cell lines tested to date in vitro and in vivo and at higher concentrations they have induced caspase-independent cell death [67,68,69]. Also, the DIFs induce differentiation of murine and human leukemia (B8 and K562) cells in vitro [61,64] and promote retinoic acid-induced differentiation of human leukemia HL-60 cells in vitro [63]. In addition, the DIFs suppress the migration of some cancer cells in vitro and in vivo [72,82]. Chemical structure–activity relationship analyses have revealed that DIF-3(+1) and Bu-DIF-3 are promising lead compounds for the development of anti-cancer drugs [72,73,74].