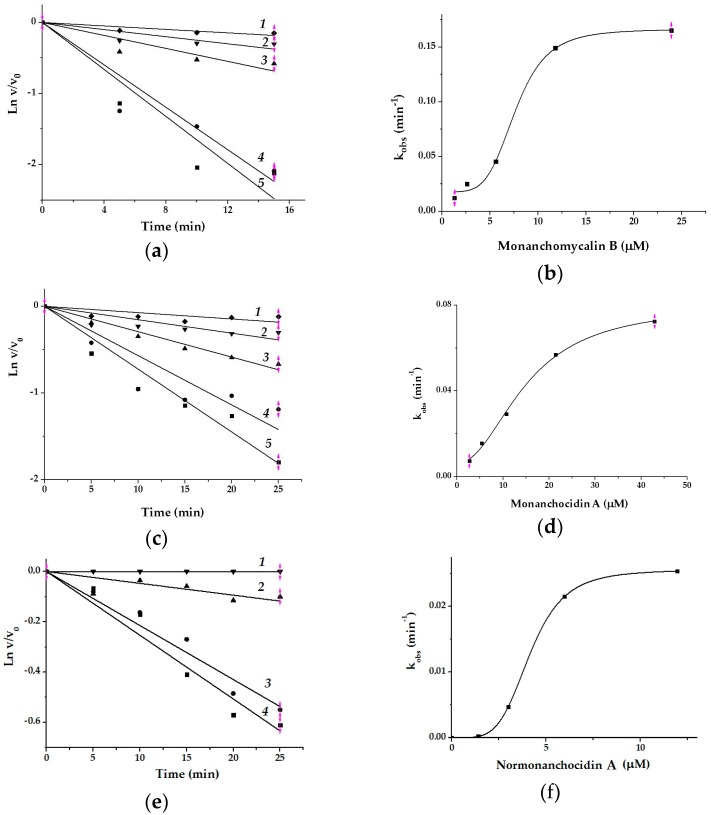
Figure 2.
The results of kinetic studies of the α-PsGal inactivation by pentacyclic guanidine alkaloids: (a) the kinetic change of the residual activity of the enzyme (v/v0) in semilogarithmic coordinates at 1.3 μM (1), 2.66 μM (2), (3) 5.69 μM, (4) 11.8 μM, and (5) 23.9 μM of monanchomycalin B; (b) the inactivation rate constants (kobs) dependence on the concentrations of monanchomycalin B; (c) the kinetic change of the residual activity of the enzyme in semilogarithmic coordinates at 2.7 μM (1), 5.4 μM (2), (3) 10.7 μM, (4) 21.4 μM, and (5) 42.9 μM of monanchocidin A; (d) the inactivation rates (kobs) dependence on the concentrations of monanchocidin A; (e) the kinetic change of the residual activity of the enzyme in semilogarithmic coordinates at 1.49 μM (1), 2.98 μM (2), 5.97 μM (3), and 11.9 μM (4) of normonanchocidin A; (f) the inactivation rates (kobs) dependence on the concentrations of normonanchocidin A. All of the experiments were performed in duplicates.