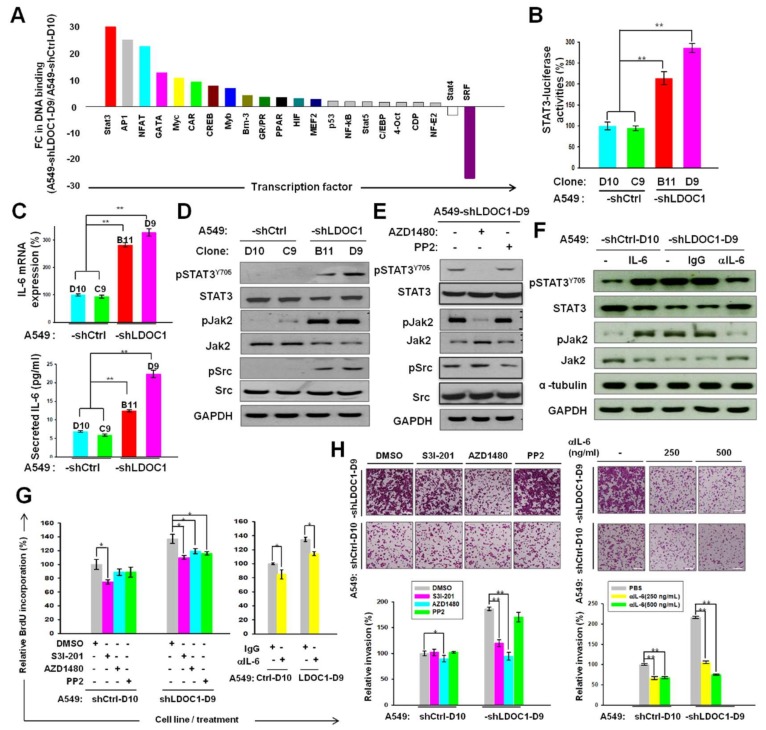
Figure 4.
Silence of LDOC1 induced a reciprocal activation loop of IL-6/JAK2/STAT3, through which LDOC1 mediated cell cycle progression and invasiveness of lung cancer cells. (A) LDOC1 modulates DNA binding activities of several transcription factors (TFs) in A549 cells. TF activation profiling assays were performed with nuclear extracts of A549-shLDOC1-D9 and A549-shCtrl-D10 cells. The values derived from TFII were set as 1 and used for normalization. The fold change (FC) of TF DNA binding activities in A549-shLDOC1-D9 versus A549-shCtrl-D10 was calculated using the average of two independent experiments. (B,C) LDOC1 expression regulates the transcriptional activities of STAT3 (B, luciferase reporter assay) and IL-6 mRNA expression (C, upper, qPCR) and secretion (C, bottom, enzyme-linked immunosorbent assay). Data are presented as mean ± SD (n ≥ 3), analyzed using a Student’s t-test, where ** p < 0.01 versus controls. (D) LDOC1 knockdown induces phosphorylation of STAT3Y705, JAK2, and Src in A549-shLDOC1-B11 and -D9 clones. (E) Tyrosine kinase activity of JAK2, not Src, is required for phosphorylation of STAT3Y705 in A549-shLDOC1-D9 cells. (F) Autocrine IL-6 signaling is involved in phosphorylation of JAK2 and STAT3Y705 in A549-shLDOC1-D9 cells. Cellular protein lysate was harvested after treatment with inhibitors of JAK2 (AZD1480, 0.5 μM), Src (PP2, 5 μM) (E), recombinant human IL-6 (100 ng/mL), or IL-6 neutralizing antibodies (250 ng/mL) (F) for 4 h, followed by Western blotting analysis of pSTAT3Y705, STAT3, pJAK2, JAK2, GAPDH, and α-tubulin. Cells treated with DMSO (0.1%, E) or IgG (250 ng/mL, F) were used as controls. Cell cycle progression (BrdU incorporation) (G) and trans-well invasiveness (H) ofA549-shCtrl-C9 and A549-shLDOC1-D9 were assessed in the presence or absence ofS3I-201 (10 μM), AZD1480 (0.5 μM), or PP2 (5 μM) or indicated concentrations of IL6-neutralizing antibodies. The invasiveness was determined and quantified as described in Figure 3. Representative photographs (upper) and quantification (bottom) of invading cells are shown. Scale Bar, 1 mm. Data are presented as mean ± SD, obtained from four independent experiments, where * p < 0.05 and ** p < 0.01.