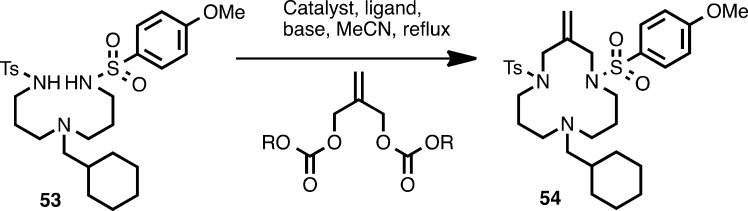
Table 3. Yield Optimization for Synthesis of VGD020 (54)a.
| catalyst (equiv) | ligand (equiv) | base (equiv) | R | yield (%) |
|---|---|---|---|---|
| Pd2dba3 (0.03) | dppb (0.06) | none | tBu | variable |
| Pd2dba3 (0.03) | dppb (0.06) | Na2CO3 (0.1) | tBu | 25–26 |
| Pd2dba3 (0.03) | dppb (0.06) | Na2CO3 (1.0) | tBu | 25–26 |
| Pd(OAc)2 (0.03) | dppb (0.06) | Na2CO3 (1.0) | tBu | 20 |
| Pd(PPh3)4 (0.06) | dppb (0.06) | Na2CO3 (1.0) | tBu | 10 |
| Pd2dba3 (0.03) | none | Na2CO3 (1.0) | tBu | 0 |
| Pd2dba3 (0.03) | PPh3 (0.06) | Na2CO3 (1.0) | tBu | 20 |
| Pd2dba3 (0.03) | dppf (0.06) | Na2CO3 (1.0) | tBu | 9 |
| Pd2dba3 (0.03) | dppb (0.06) | Na2CO3 (1.0) | Me | 40 |
| Pd2dba3 (0.03) | dppb (0.06) | Na2CO3 (1.0) | tBu | 50–56b |
| Pd2dba3 (0.03) | dppb (0.06) | Na2CO3 (1.0) | tBu | 48c |
Reaction conditions (unless indicated otherwise): 15–20 mM disulfonamide in anhydrous acetonitrile, stirred under reflux for 18–24 h.
9 mM disulfonamide.
4 mM disulfonamide.