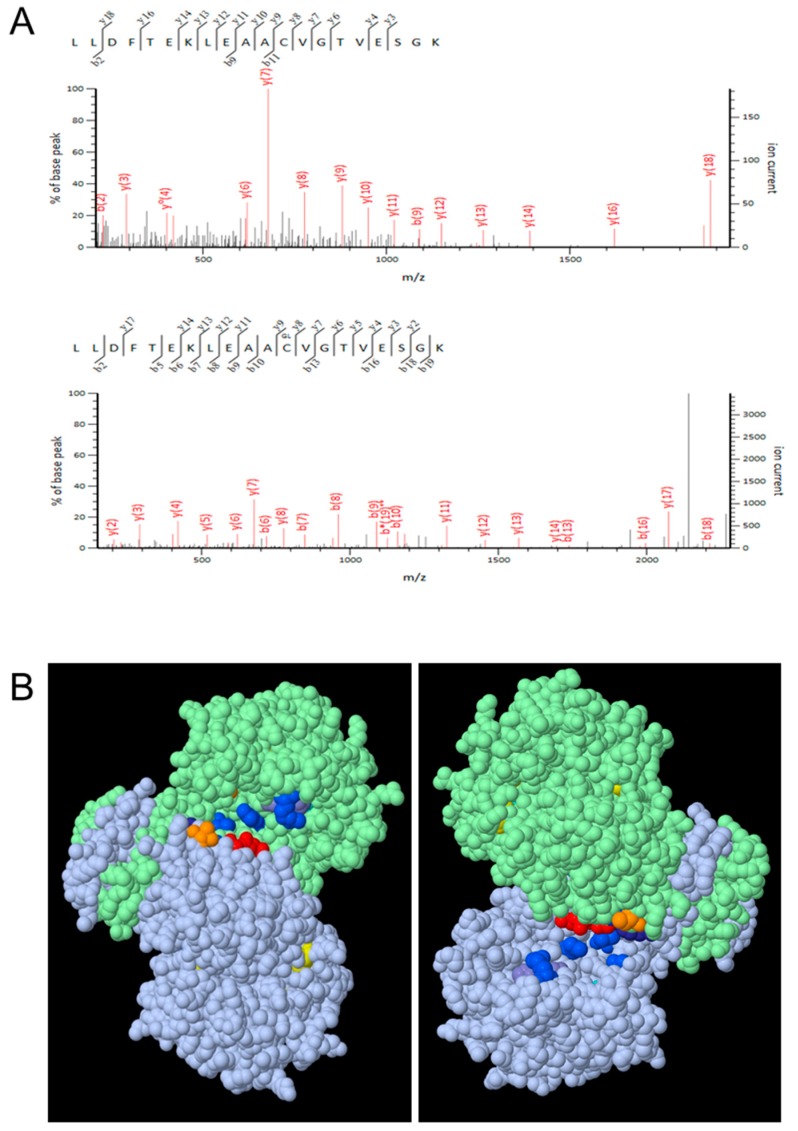
Figure 3.
Glutathionylation of cICDH. (A) cICDH was treated or not with 1 mM GSNO for 30 min at 25 °C. Samples were trypsin digested and analyzed by nanoLC-MSMS. The panels show fragmentation spectra matching peptides with either unmodified (top) or with glutathionylated C363 (bottom). The same glutathionylated residue was identified in three biological repetitions. (B) Modeled cICDH (residues 4–408) homodimer (grey and green chains) based on human cytosolic ICDH. Conserved amino acids (R111, R134, Y141, T214, D252, D279, R314, H315) with the most plant and mammalian NADP-ICDH proteins, in the active site pocket are shown in different colors depending on the nature of the residue: R, blue; Y, purple; T, orange; D, red; H, grey. The conserved cysteine C363 in each chain is represented in yellow (arrowheads). In the right panel, the cICDH homodimer was rotated 180°.