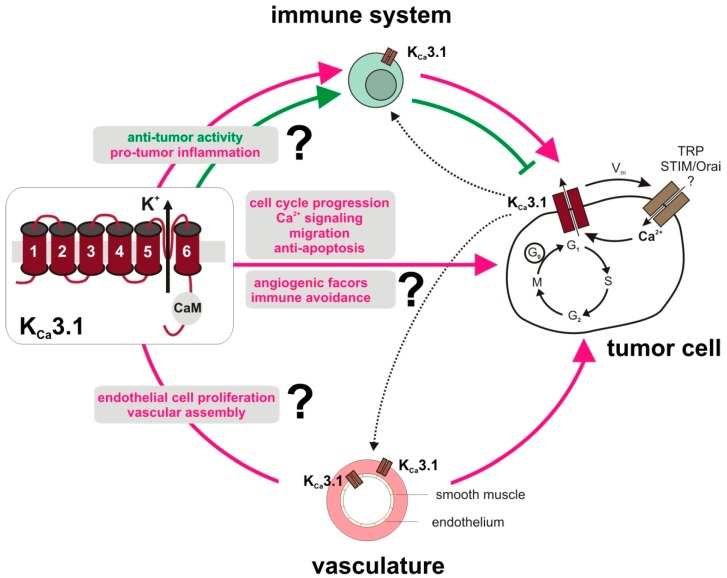
Figure 3.
Role of the KCa3.1 channel in tumor-associated cells. Tumors from different entities and various microenvironmental cell types, i.e., immune cells, vasculature, fibroblasts (not shown) express functional KCa3.1 channels. Physiological roles and tumor behaviors of KCa3.1 are cell type-dependent, but involve proliferation, migration and cancer progression. KCa3.1 channel expression seems to be a crucial determinant of cancer risk and, in established cancers, KCa3.1 upregulation at the end of G1 phase of the cell cycle was seen in various tumor cell types [36]. By its interaction with [Ca2+]i via its constitutively bound calmodulin (CaM), with other ion channels such as TRP or STIM/Orai, with changes in the membrane potential (Vm), and with apoptotic pathways, KCa3.1 may further contribute to aberrant tumor cell signaling. Beyond that, tumor-promoting KCa3.1 activity in stromal cells has been described. Several studies find evidence for KCa3.1 expression in endothelial and in activated smooth muscle cells of the vasculature pointing to its role in tumor angiogenesis and/or vasculogenesis. Moreover, growth factor signaling was linked to KCa3.1 in fibroblasts to promote epithelial-mesenchymal transition in breast cancer (not depicted) [84]. Proper activation and function of various immune cell subsets requires KCa3.1. Therefore, perturbed KCa3.1 signaling may prevent cancer progression and disturb e.g., the immune cell´s pro-angiogenic program, but also its activity to recognize and eliminate tumor cells. Apparently, the impact of a tumor and stromal versus immune cell KCa3.1 inhibition on tumor progression and therapy success and thus also interaction between the different cell types, as indicated by dotted lanes, requires further investigations.