Abstract
Background
To study the antidiabetic effects and mechanisms of the fenugreek extracts in streptozotocin (STZ)‐induced type 2 diabetic (T2DM) mice fed a high‐fat diet (HFD).
Methods
We established C57BL/6J mice model of T2DM using HFD‐fed and STZ‐induced method. Then, the mice were administered with two types of fenugreek extracts (E1, flavonoid and E2, stilbene glycoside) for 4 weeks and the effects on fasting blood glucose (FBG), weight, superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), and pathological indexes were investigated.
Results
Administration of fenugreek extracts decreased the FBG level compared with that of the model group. Comparatively, the high‐dose E2 decreased the FBG more significantly than the other treatments did. Both extracts showed an obvious antioxidant effect by increasing serum SOD and CAT activities and decreasing the MDA content. Furthermore, the high‐dose E1 showed a significant difference (P < .01) compared with the model group in the three investigated indexes.
Conclusion
Our study demonstrated that both the flavonoid and stilbene glycoside extracts of fenugreek improved the hyperglycemia in the T2DM mice model. Moreover, the antidiabetic effects of both extracts might be due to their antioxidant activity in vivo.
Keywords: C57BL/6J mice, diabetes mellitus, fenugreek, hyperglycemia
1. INTRODUCTION
Diabetes mellitus (DM) is prevalent worldwide, and according to World Health Organization (WHO) and the American Diabetes Association diagnostic criteria, 439 million adults will be affected by diabetes by 2030.1 DM is characterized by a relative or absolute lack of insulin, which may lead to hyperglycemia. The two main types of DM are type 1 DM (T1DM) and type 2 DM (T2DM). T1DM is an autoimmune disease that leads to the destruction of the insulin‐producing pancreatic β‐cells in the islets, and it is most commonly diagnosed in children and young adults. T2DM is a complex, heterogeneous, and polygenic disease characterized mainly by insulin resistance and pancreatic β‐cell dysfunction. It is the most common type of diabetes, and the percentage rate is 90% of all incidences DM.2, 3 Some data indicate that high‐fat diet (HFD) and obesity are the main risk factors for T2DM.4 Therefore, in theory, weight reduction and exercise should improve the T2DM state, but in reality, lifestyle interventions have failed in numerous cases. Therefore, drugs must be used in these T2DM cases to avoid the deleterious effects of chronic hyperglycemia. Currently, T2DM drug treatments mainly include chemical drugs such as biguanides, thiazolidinediones, sulfonylureas, d‐phenylalanine, meglitinides, and α‐glucosidase inhibitors in addition to insulin. All these drugs have different degrees and types of side effects5, 6; therefore, there is a demand for new safer compounds for the treatment of diabetes. Because of the apparent minimal side effects, natural products have attracted the attention of scientists.
Fenugreek (Trigonella foenum‐graecum L), a well‐known annual herb belonging to the family Leguminosae, has been widely used as a spice, edible vegetable, and medicinal plant for a long time. Furthermore, some studies have reported on the active components of fenugreek.7, 8, 9, 10, 11 4‐Hydroxyisoleucine and galactomannan, isolated from fenugreek, can lower the blood glucose levels and improve lipid metabolism in vivo.12 Other studies have suggested that fenugreek might possess antihypoglycemic and antihyperlipidemic effects in humans and animal models.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 The mechanism of antihypoglycemia induced by fenugreek seed extract was shown to be mediated through stimulation of an insulin signaling pathway in a research study.17
2. MATERIALS AND METHODS
2.1. Preparation of fenugreek
Fenugreek was collected from Qinghai Province of the PR of China, where Chinese herbal medicine is abundant. The purified fenugreek extract was obtained from Northwest Institute of Plateau Biology, Chinese Academy of Sciences in December 2015. Dried fenugreek seeds (500 g) were ground into a powder and sequentially extracted thrice with 75% ethanol (10 L) under reflux at 60°C. After vacuum concentration, 245 g of the residues was diluted with water (1 L) and further successively extracted with light petroleum (boiling point 60‐90°C, 2.0 L), ethyl acetate (2 L), and n‐butanol (3 L). Each solution was vacuum evaporated to dryness at 60°C to obtain a total yield of 30.5, 33.7, and 35 g of petroleum ether, ethyl acetate (flavonoid), and n‐butanol (stilbene glycoside) extracts, respectively.
2.2. Study design
Male C57BL/6J mice (n = 100, Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) weighing 18‐22 g (4 weeks age) were purchased and maintained at 5 animals per IVC cage at a stable temperature (22‐23°C) and humidity (60%), with a 12‐h light‐dark cycle in the Laboratory Animal Center Chinese Center for Diseases control and Prevention, Beijing. The animal had free access to water and food. After acclimation for 1 week, the mice were fed HFD (the ingredients were consistent with the D12492 diet) except for the uninduced control. Then, 5 weeks later, they were injected with STZ to induce T2DM, and 72 h later, the fasting blood glucose (FBG) was measured. Mice with FBG levels ≥ 11 mmol/L were considered hyperglycemic in our study. According to the blood glucose level, 60 mice were randomly divided into the following 6 groups: positive control (PC), T2DM animal model (M), flavonoid high and low doses (E1H and E1L, respectively), and stilbene glycoside high and low doses (E2H and E2L, respectively). Fenugreek extracts at doses of 20 and 80 mg kg−1 d−1 were intragastric administrated to the EH and EL groups, respectively, for 4 weeks. Metformin (500 mg kg−1 d−1) was used as a positive control.
The FBG of the mice was tested weekly, and at the end of week 4 after intragastric administration of the test substances, the antioxidant and pathological indexes were assayed. This study was approved and supervised by the Laboratory Animal Welfare and Ethics Committee in China CDC (permission no. 2016‐CCDC‐WER‐002).
2.3. Measurement of serum parameters
After 4 weeks of fenugreek extract treatment, the mice were fasted overnight, and blood samples were drawn from the tail tip to determine the FBG using a glucose meter. Then, more blood samples were obtained from the orbital venous plexus for serum parameter analysis. The serum concentrations of glucose, malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) were determined using a spectrophotometer (Spectra MRTM, Dynex Technologies, Chantilly, Virginia, USA) and commercial assay kits (Nanjing Jian Cheng Biology Co., Nanjing, China).
2.4. Pathological indexes
After blood collection, the mice were dissected after euthanasia, and the liver, pancreas, and kidney were fixed in 10% formalin buffer for the pathological indexes determination, which was conducted by the College of Veterinary Medicine, China Agricultural University.
2.5. Statistical analyses
The data were expressed as means ± standard error of the mean (SEM). The statistical package for the social sciences (spss) for Windows version 19 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. The Mann‐Whitney U and Kruskal‐Wallis tests were performed. The results of the nonparametric comparisons were considered statistically significant at P < .05.
3. RESULTS
3.1. Two main effective constituents of fenugreek
The high‐performance liquid chromatography (HPLC) product analysis chromatogram is shown in Figure 1. All the above experiments were performed at the Northwest Institute of Plateau Biology, Chinese Academy of Sciences.
Figure 1.
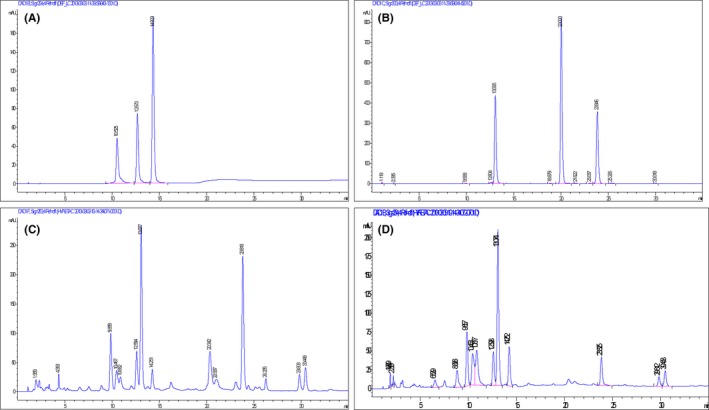
High‐performance liquid chromatography (HPLC) product chromatograms. A, Three standard substance (flavonoids), orientin (retention time [RT] = 10 min), vitexin (RT = 12 min), and isovitexin (RT = 14 min). B, Three standard substance (stilbene glycosides), rhaponticin (RT = 13 min), deoxyrhapontin (RT = 20 min), and leaves simple emodin (RT = 23 min). C, Fenugreek ethyl acetate extract. D, Fenugreek 30% n‐butyl alcohol extracts
3.2. Blood glucose level and weight
After the STZ injection, the blood glucose level and weight were measured once a week. The body weight declined by week 4 for the M, PC, E1H, E1L, E2H, and E2L groups was 17.1%, 20.8%, 14.4%, 15.9%, 11.2%, and 11.3%, respectively, compared to the weight at week 1 (Table 1). The body weight and blood glucose level were measured on the same day. The M group displayed a glucose level of 17.92 ± 0.97 mmol/L g at week 1, which was almost invariant up to week 4 when it was 17.68 ± 3.69 mmol/L. In contrast, the glucose level was significantly (P < .01) reduced at 19.2%, and 36.5% by week 4 in the E1H and E2H group compared to their respective values at week 1 (Table 2).
Table 1.
Change in weight of type 2 diabetes mellitus (T2DM) mouse model
| Group/Week | Weight (g) | |||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | |
| M Gr. | 21.00 ± 5.00 | 18.20 ± 3.70 | 19.00 ± 2.74 | 17.40 ± 2.97 |
| PC Gr. | 25.83 ± 2.83 | 24.00 ± 2.00 | 23.67 ± 2.25 | 22.83 ± 2.93 |
| E1H Gr. | 25.00 ± 5.10 | 22.00 ± 4.69 | 21.60 ± 3.51 | 21.40 ± 4.72 |
| E1L Gr. | 26.40 ± 4.51 | 22.20 ± 3.56 | 22.00 ± 3.81 | 22.20 ± 5.07 |
| E2H Gr. | 23.20 ± 3.63 | 21.00 ± 2.33 | 21.40 ± 2.07 | 20.60 ± 3.29 |
| E2L Gr. | 23.00 ± 3.16 | 21.8 ± 3.77 | 22.20 ± 3.42 | 20.40 ± 6.02 |
n = 10; M, T2DM model; PC, positive control; E1H and E1L, flavonoid high and low doses, respectively; E2H and E2L, stilbene glycoside high and low doses, respectively.
Table 2.
Change in blood glucose level of type 2 diabetes mellitus (T2DM) mouse model
| Blood glucose level (mmol/L) | ||||
|---|---|---|---|---|
| Group/Week | Week 1 | Week 2 | Week 3 | Week 4 |
| M | 17.92 ± 0.97 | 17.12 ± 3.57 | 16.82 ± 3.67 | 17.68 ± 3.69 |
| PC | 15.00 ± 4.30 | 16.50 ± 4.95 | 10.00 ± 5.05a | 10.75 ± 4.35a |
| E1H | 16.50 ± 6.03 | 16.25 ± 5.38 | 13.67 ± 3.06a | 13.33 ± 6.43a |
| E1L | 16.20 ± 3.70 | 15.60 ± 4.83 | 16.00 ± 3.16b | 16.50 ± 7.93 |
| E2H | 17.33 ± 1.53 | 13.33 ± 4.04a | 13.33 ± 2.08a | 11.00 ± 1.60a |
| E2L | 17.00 ± 3.00 | 18.40 ± 1.95 | 16.00 ± 2.55b | 16.20 ± 5.76b |
n = 10; M, T2DM model; PC, positive control; E1H and E1L, flavonoid high and low doses, respectively; E2H and E2L, stilbene glycoside high and low doses, respectively.
P < .01 compared with week 1 value of respective group.
P < .05 compared with week 1 value of respective group.
3.3. Estimation of SOD and CAT activity
The serum SOD activity declined in the M group and ameliorated in E1H and E2H groups compared with that in the normal group. The increase in SOD activity of the E1H and E2H groups was 27.6% and 25.0%, respectively (Table 3) compared to that of the model group (P < .01) and similar results were obtained with the CAT activity analysis. The increase in CAT activity of the E1H and E2H groups was 39.0% and 40.6%, respectively (Table 3) compared to the model group (P < .01).
Table 3.
Change in serum antioxidant levels of type 2 diabetes mellitus (T2DM) mouse model
| Group/Index | SOD (IU/mL) | MDA (nM/mL) | CAT (IU/mL) |
|---|---|---|---|
| Normal | 98.43 ± 9.03 | 4.75 ± 0.35 | 68.42 ± 9.15 |
| M | 69.66 ± 3.93a | 12.57 ± 1.0 | 37.17 ± 7.21a |
| PC | 89.51 ± 2.06b | 7.41 ± 0.95b | 59.81 ± 9.60b |
| E1H | 88.86 ± 2.74b | 7.80 ± 1.16b | 51.67 ± 8.61b |
| E1L | 62.44 ± 8.47 | 9.83 ± 1.04 | 45.05 ± 8.60 |
| E2H | 87.08 ± 6.54b | 7.97 ± 1.24b | 52.25 ± 4.30b |
| E2L | 69.21 ± 1.38 | 7.74 ± 1.21b | 44.62 ± 6.28 |
P < .01, compared with normal group.
P < .01 compared with M group; n = 10; M, T2DM model; PC, positive control; E1H and E1L, flavonoid high and low doses, respectively; E2H and E2L, stilbene glycoside high and low doses, respectively.
3.4. Measurement of lipid peroxidation
The serum MDA level was increased significantly in the M group compared to the Normal group. After administration of the fenugreek extracts, the serum MDA decreased especially in the E1H, E2H, and E2L groups. Furthermore, the percentage decrease in the MDA contents was 37.9%, 36.6%, and 38.4%, respectively, compared to the M group (Table 3).
3.5. Histopathology of kidney, liver, and pancreas
The histopathological evaluation of the kidney, pancreas, and liver tissues from the control, T2DM, and fenugreek extract‐treated groups revealed obvious fatty degeneration of liver and kidney in the T2DM group. An increased number of vacuolated cells occurred in the liver and kidney tissues. The volume of the islet varied in size and fatty infiltration occurred in the T2DM group. In contrast, the degree of fatty degeneration was improved in the fenugreek extract‐treated groups, especially the E2H group, as shown in Table 4 and Figure2.
Table 4.
Fatty degeneration of liver and kidney
| Group | Blank | M | E1L | E1H | E2L | E2H | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Fatty degeneration in liver | N | N | +++ | +++ | N | N | + | N | N | + | N+ | N |
| Fatty degeneration in kidney | N | N | +++ | ++ | + | N | + | + | N | + | N | N |
N, no abnormity; +, slight lesioning; ++, no remarkable lesioning; +++, remarkable lesioning. M, T2DM model; E1H and E1L, flavonoid high and low doses, respectively; E2H and E2L, stilbene glycoside high and low doses, respectively.
Figure 2.
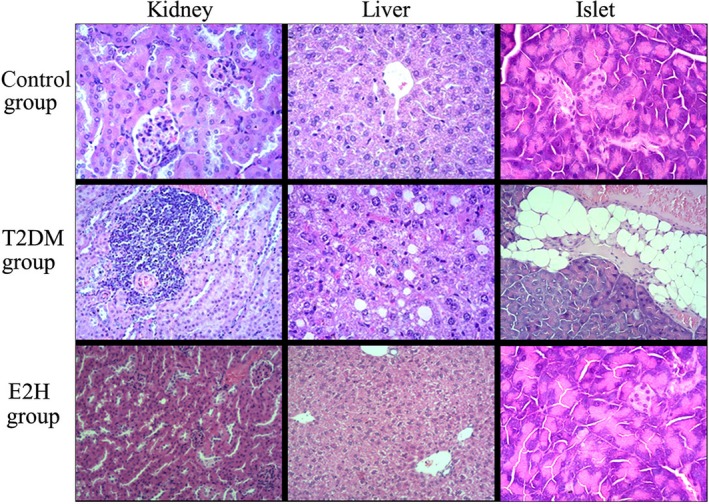
Histopathological changes in kidney, liver, and pancreas tissues stained with hematoxylin and eosin (H&E, ×200)
4. DISCUSSION
Previous studies have indicated that C57BL/6J mice are more liable to show an increase in body weight after feeding HFD, and they carry a genetic predisposition to developing T2DM.24, 25 STZ was initially isolated from Streptomyces achromogenes in 1960, but its diabetogenic properties were not described until 1963.26 Furthermore, a subsequent study indicated that the diabetogenic effects were due to selective destruction of the pancreatic islet β cells.27 In recent years, numerous researchers have used HFD and STZ‐treated rodents as T2DM animal models.2, 28, 29 The HFD can induce insulin resistance, glucose intolerance, or both, and this may mimic the human lifestyle.
In addition, STZ can shorten the time required to establish the model and improve the response of model animals. Moreover, the known characteristics of the HFD/STZ rodent model have been reviewed and compared with the pathophysiology of human T2DM.30 In our study, we verified that T2DM could be induced successfully by administering an HFD and STZ (100 mg/kg) injected into C57BL/6J mice. Table 1 shows that the blood glucose was maintained at a high level and the body weight continued to decline in the M group during the period of our study. These characteristics are in accordance with human T2DM.
In contrast, the blood glucose reduced gradually, and the weight declined slightly or remained relatively stable in the groups intragastrically administered the fenugreek extracts. Furthermore, after a 4‐week treatment, the blood glucose level was obviously lower than it was at the beginning of treatment and the effect was obviously dose‐dependent. There was a significant difference between the M and E2H groups, indicating that the E2 extracts likely had more potent effects on the hyperglycemic T2DM animal model than the other treatments did.
Currently, increasing evidence indicates that reactive oxygen species (ROS) play an important role in the developmental process of DM and its complications.31 Numerous clinical cases have also shown that a close association exists between the progression of DM and oxidative stress, which promotes the DM process when ROS levels increase or oxidative damage occurs.32 Free radicals can cause polyunsaturated fatty acid peroxidation and the final product, MDA, can generate protein, nucleic acid, and lipid cross‐linking, and biological membrane denaturation, as well as cell mutation, aging, and death. The MDA level reflects the degree of lipid peroxidation in vivo, and indirectly reflects the degree of cell damage.33 The SOD enzyme can eliminate free radicals directly, interdict chain reactions of the free radicals, and then protect the cells against destruction by these processes.34
By reacting with hydrogen peroxide (H2O2), glutathione (GSH) can maintain the structural integrity of the cell membrane. Low levels or depletion of GSH induces numerous chemical substances or aggravates the effects of toxic environmental factors. This may be related to the increase in oxidative damage. Therefore, the level of GSH is an important factor for measuring the antioxidant ability of an organism. In our study, we observed a remarkable increase in serum MDA levels of the M group mice, with a decline in the SOD activity. These results indicate that the increase in free radical levels enhanced the lipid peroxidation in the body of M group mice. This reflected the declining antioxidant ability of the body. In contrast, the E1H‐ and E2H‐treated groups showed adverse effects. The amount of MDA was decreased while the SOD and CAT activity increased compared to that of the M group. All the results indicate that fenugreek extracts may improve SOD activity, clear oxygen radicals, and inhibit lipid peroxidation in the body. Especially, administration of the high dose of the extracts induced significant effects on these indexes.
The histopathological changes in the kidney, liver, and pancreas tissues indicated that there was serious fatty degeneration of the kidneys and livers of the M group mice. In contrast, no abnormal changes were observed in the blank control group. The groups administered fenugreek extracts showed an improvement in the degree of fatty degeneration.
The size of islet cells in the M group was considerably different from that of the other groups. The islets in the blank control group were obviously smaller than those in the M group, and the same effect was observed with the number of islets.
5. CONCLUSION
The results revealed that this animal model of T2DM was successfully established and suitable for this study. The comparison of the antioxidant indexes (SOD, MDA, and CAT) of the mice in the M group and those cured by treatment with the fenugreek extracts revealed that flavonoids and stilbene glycosides were the two main active components that mediated the antidiabetic effects in the T2DM mice model.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All listed authors meet the requirements for authorship. XYL was in charge of the animal model experiment and wrote the article. SSL did the animal model experiment and other tests. HLW designed the animal experiment and modified the article. GL analyzed the results of the animal model experiment. YFH completed the purification of Fenugreek. XYL assisted with the biochemistry tests. RR assisted with the animal experiment. JL gave article writing suggestions. XCL gave the idea of the article. All authors read and approved the final manuscript.
Li XY, Lu SS, Wang HL, et al. Effects of the fenugreek extracts on high‐fat diet‐fed and streptozotocin‐induced type 2 diabetic mice. Anim Models Exp Med. 2018;1:68–73. 10.1002/ame2.12004
Xiao‐yan Li and Shuang‐shuang Lu contributed equally to this work.
REFERENCES
- 1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 2. King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salas‐Salvadó J, Martinez‐González MÁ, Bulló M, Ros E. The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21:B32–B48. [DOI] [PubMed] [Google Scholar]
- 4. Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. [DOI] [PubMed] [Google Scholar]
- 5. U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 6. Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–827. [DOI] [PubMed] [Google Scholar]
- 7. Handa T, Yamaguchi K, Sono Y, Yazawa K. Effects of fenugreek seed extract in obese mice fed a high‐fat diet. Biosci Biotechnol Biochem. 2005;69:1186–1188. [DOI] [PubMed] [Google Scholar]
- 8. Ajabnoor MA, Tilmisany AK. Effect of Trigonella foenum graceum on blood glucose levels in normal and alloxan‐diabetic mice. J Ethnopharmacol. 1988;22:45–49. [DOI] [PubMed] [Google Scholar]
- 9. Abdel‐Barry JA, Abdel‐Hassan IA, Al‐Hakiem MH. Hypoglycaemic and antihyperglycaemic effects of Trigonella foenum‐graecum leaf in normal and alloxan induced diabetic rats. J Ethnopharmacol. 1997;58:149–155. [DOI] [PubMed] [Google Scholar]
- 10. Khosla P, Gupta DD, Nagpal RK. Effect of Trigonella foenum graecum (Fenugreek) on blood glucose in normal and diabetic rats. Indian J Physiol Pharmacol. 1995;39:173–174. [PubMed] [Google Scholar]
- 11. Xue WL, Li XS, Jian Z, Liu YH, Wang ZL, Zhang RJ. Effect of Trigonella foenum‐graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin‐induced diabetic rats. Asia Pac J Clin Nutr. 2007;16:422–426. [PubMed] [Google Scholar]
- 12. Basch E, Ulbricht C, Kuo G, Szapary P, Smith M. Therapeutic applications of fenugreek. Altern Med Rev. 2003;8:20–27. [PubMed] [Google Scholar]
- 13. Sharma RD. Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects. J Ethnopharmacol. 1986;6:1353–1364. [Google Scholar]
- 14. Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr. 1990;44:301–306. [PubMed] [Google Scholar]
- 15. Sharma RD, Sarkar A, Hazara DK, et al. Use of fenugreek seed powder in the management of non‐insulin dependent diabetes mellitus. Nutr Res. 1996;16:1331–1339. [Google Scholar]
- 16. Bin‐Hafeez B, Haque R, Parvez S, Pandey S, Sayeed I, Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int Immunopharmacol. 2003;3:257–265. [DOI] [PubMed] [Google Scholar]
- 17. Vijayakumar MV, Singh S, Chhipa RR, Bhat MK. The hypoglycaemic activity of fenugreek seed extract is mediated through the stimulation of an insulin signalling pathway. Br J Pharmacol. 2005;146:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hannan JM, Ali L, Rokeya B, et al. Soluble dietary fibre fraction of Trigonella foenum‐graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br J Nutr. 2007;97:514–521. [DOI] [PubMed] [Google Scholar]
- 19. Eidi A, Eidi M, Sokhteh M. Effect of fenugreek (Trigonella foenum‐graecum L) seeds on serum parameters in normal and streptozotocin‐induced diabetic rats. Nutr Res. 2007;27:728–733. [Google Scholar]
- 20. Mowla A, Alauddin M, Rahman MA, Ahmed K. Antihyperglycemic effect of Trigonella foenum‐graecum (fenugreek) seed extract in alloxan‐induced diabetic rats and its use in diabetes mellitus: a brief qualitative phytochemical and acute toxicity test on the extract. Afr J Tradit Complement Altern Med. 2009;6:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshinari O, Igarashi K. Anti‐diabetic effect of trigonelline and nicotinic acid, on KK‐A(y) mice. Curr Med Chem. 2010;17:2196–2202. [DOI] [PubMed] [Google Scholar]
- 22. Hamza N, Berke B, Cheze C, et al. Preventive and curative effect of Trigonella foenum‐graecum L. seeds in C57BL/6J models of type 2 diabetes induced by high‐fat diet. J Ethnopharmacol. 2012;142:516–522. [DOI] [PubMed] [Google Scholar]
- 23. Haber SL, Keonavong J. Fenugreek use in patients with diabetes mellitus. Am J Health Syst Pharm. 2013;70:1202–1203. [DOI] [PubMed] [Google Scholar]
- 24. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet‐induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. [DOI] [PubMed] [Google Scholar]
- 25. Jang A, Srinivasan P, Lee NY, et al. Comparison of hypolipidemic activity of synthetic gallic acid‐linoleic acid ester with mixture of gallic acid and linoleic acid, gallic acid, and linoleic acid on high‐fat diet induced obesity in C57BL/6 Cr Slc mice. Chem Biol Interact. 2008;174:109–117. [DOI] [PubMed] [Google Scholar]
- 26. Rakieten N, Rakieten ML, Nadkarni MV. Studies on the diabetogenic action of streptozotocin (NSC‐37917). Cancer Chemother Rep. 1963;29:91–98. [PubMed] [Google Scholar]
- 27. Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967;126:201–205. [DOI] [PubMed] [Google Scholar]
- 28. King A, Bowe J. Animal models for diabetes: understanding the pathogenesis and finding new treatments. Biochem Pharmacol. 2016;99:1–10. [DOI] [PubMed] [Google Scholar]
- 29. Skovso S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014;5:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Islam MS, du Loots T. Experimental rodent models of type 2 diabetes: a review. Methods Find Exp Clin Pharmacol. 2009;31:249–261. [DOI] [PubMed] [Google Scholar]
- 31. Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose‐induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 32. Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species‐regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S241–S245. [DOI] [PubMed] [Google Scholar]
- 33. Wallace DC. Animal models for mitochondrial disease. Methods Mol Biol. 2002;197:3–54. [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto H, Zhao P, Inoue K. Origin of two isoprenoid units in a lavandulyl moiety of sophoraflavanone G from Sophora flavescens cultured cells. Phytochemistry. 2002;60:263–267. [DOI] [PubMed] [Google Scholar]


