Introduction
Classically, several therapeutic options have been described to treat patients affected by hidradenitis suppurativa (HS). Adalimumab is the only biologic approved by the US Food and Drug Administration available for therapy of moderate-to-severe HS. However, treatment may not always get the desired results, and adverse events may be possible. Alternative therapies are being studied for HS, including treatments using the interleukin (IL)-17A inhibitor.1 We report the potential double pathogenetic face of secukinumab in HS, describing a case of secukinumab-induced HS in a psoriasis patient successfully treated with adalimumab, and a case of HS and related psoriasiform eruption caused by adalimumab treatment, controlled by secukinumab therapy.
Case 1
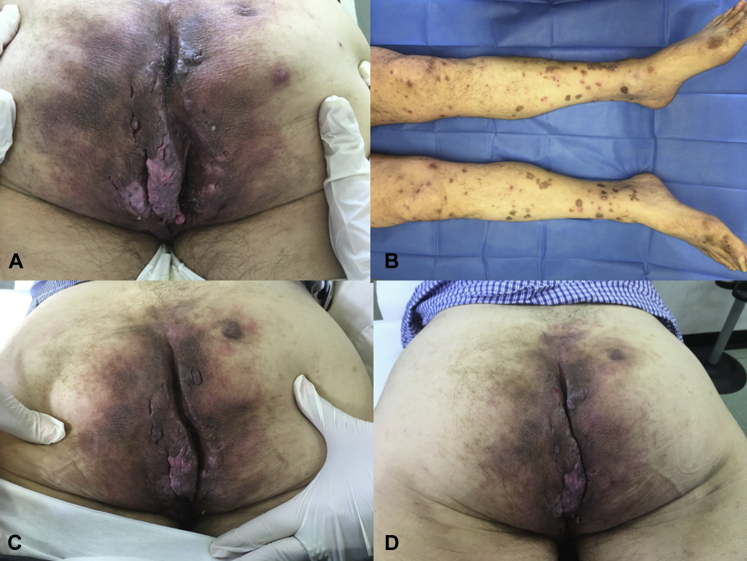
A 63-year-old man suffering from HS since 2008 was admitted to our clinic in August 2017. On examination, inflammatory papules, nodules, abscesses, and fistulae were observed in the perineal area and the buttocks (Fig 1, A). His HS was staged as severe (Hurley stage III). His medical history was unremarkable, except for hepatitis B, treated with entecavir, obtaining negativization of the viral load. The patient had undergone different HS treatment regimens over the years including rifampicin/clindamycin, cyclosporine, and infliximab, which provided only limited and temporary benefit. The patient also underwent a range of surgical interventions to control skin lesions. In September 2017, he started treatment with adalimumab with standard dosage for HS. HS lesions slightly improved after 8 weeks, when an erythematous-desquamative eruption located on the lower limbs appeared (Fig 1, B). The patient did not take other drugs concurrently. A skin biopsy found parakeratosis without hyperkeratosis, acanthosis with downward elongation of rete ridges, thin granular cell layer, suprapapillary thinning, and neutrophils in parakeratotic scale, confirming the diagnosis of a psoriasiform eruption. Therefore, adalimumab therapy was discontinued, and secukinumab (300 mg at weeks 0, 1, 2, 3, and 4 and then every 4 weeks) was started, achieving partial HS improvement and psoriasiform eruption resolution within the first 8 weeks of treatment (Fig 1, C and D).
Fig 1.
Patient 1. A, Clinical presentation characterized by the presence of inflammatory papules, nodules, abscesses, and fistulae in the perineal area as well as the buttocks. B, Psoriasiform eruption on adalimumab located at the lower limbs. C and D, Secukinumab therapy (300 mg at week 0, 1, 2, 3, 4 and then every 4 weeks) achieving partial HS improvement and psoriasiform eruption resolution within the first 8 weeks of treatment.
Case 2
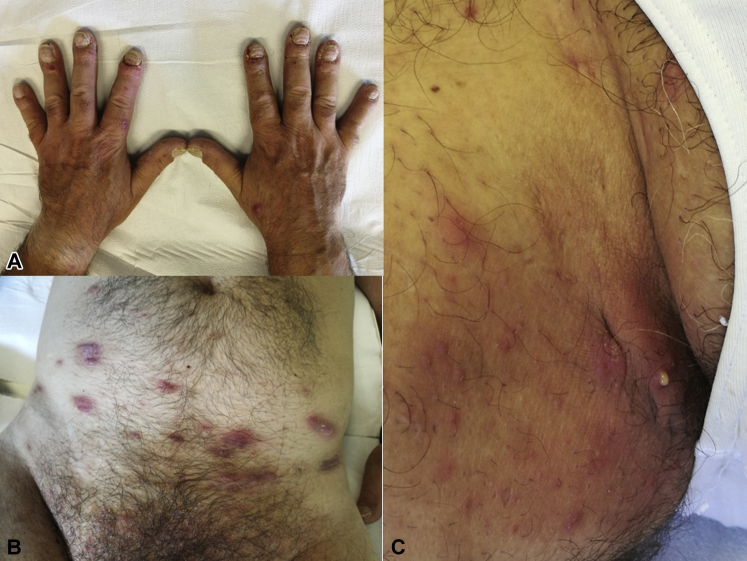
A 46-year-old man, affected by psoriasis, was admitted to our department for a routine follow-up visit in July 2017. The patient had undergone different psoriasis treatment regimens over the years including cyclosporine, methotrexate, and ustekinumab. Because of the persistence of psoriasis skin lesions, the patient started secukinumab treatment (300 mg at weeks 0, 1, 2, 3, 4 and then every 4 weeks) in November 2016 with good results. At the follow-up visit in July 2017, psoriasis skin lesions were confined to dorsal aspects of both hands, particularly on periungueal area and nails (Fig 2, A). A full dermatologic examination found the presence of multiple erythematous nodules and papules, as well as some abscesses, localized at the axillary, inguinal, and abdominal areas (Fig 2, B and C). The patient did not take other drugs concurrently. The patient related that the lesion had developed for 3 months, progressively increasing as the time went by. A skin biopsy was taken from a nodule of the abdominal region showing the presence of a hyperplastic epidermidis and a perifollicular inflammatory infiltrate with neutrophils, lymphocytes, and histiocytes. As far as his family history is concerned, his mother was affected by HS. Based on medical history and clinical and histologic examinations, a diagnosis of HS was made. Secukinumab treatment was interrupted, and a combined rifampicin plus clindamycin regimen (300 mg twice a day or 600 mg/d and 300 mg twice a day, respectively) was conducted for 6 weeks with partial clinical improvement of HS lesions. Afterward, the patient started on adalimumab treatment (160 mg at day 1, 80 mg at day 15, 40 mg at day 29, then 40 mg weekly) with a greater clinical response of HS lesions and psoriasis.
Fig 2.
Patient 2. A, Psoriasis skin lesions confined to dorsal aspects of both hands, particularly on periungueal area and nails. B and C, Presence of multiple erythematous nodules and papules, as well as some abscesses, localized at axillary, inguinal, and abdominal area.
Discussion
Adalimumab constitutes the only biologic therapy for HS approved by the US Food and Drug Administration.1 However, lack of efficacy and adverse events may also be associated with this therapy. Increased IL-17 serum concentrations has been seen in patients with HS as well as a significantly increased number of IL-17–producing cells in lesional and in perilesional HS skin compared with healthy subjects.2, 3 Therefore, IL-17 pathway may play a key role in HS pathogenesis. Currently, 4 case reports described a significant improvement of lesion activity and inflammation with secukinumab in HS patients.4, 5, 6, 7 Furthermore, secukinumab is now in phase I trial for HS treatment. We report these 2 clinical cases to highlight the possible double face of secukinumab in HS management, describing possible complete resolution of HS lesions as well as possible paradoxical reactions such as anti–IL-17–induced HS onset. In this context, a parallel between secukinumab and adalimumab can be drawn. Nevertheless, adalimumab is approved for HS and psoriasis treatment, and paradoxical cases of HS or psoriasiform eruption induced by this anti–tumor necrosis factor (TNF)-α are described in literature.8 Particularly, a multicenter nationwide retrospective study was recently published reporting a paradoxical HS under biological agents with adalimumab being responsible of 48% new HS onset cases.9 An imbalance in cytokine production, an unopposed type I interferon production, and a shift toward a helper T cell 1/helper T cell 2 profile may play a role. Particularly, for psoriasiform eruptions, it is hypothesized that TNF-α inhibition may stimulate increased maturation of plasmacytoid dendritic cells with uncontrolled production of interferon-α, favoring T-cell homing to the skin and activation of T cells to produce TNF-α and IL-17.10 This finding may explain the efficacy of anti–IL-17 on the psoriasiform eruption observed in our HS patient, as increased IL-17 levels in HS skin and serum justify anti–IL-17 efficacy in HS lesions too. On the other hand, hypotheses to explain the occurrence of HS under anti-TNF-α are scant: local modification of cytokine balance and activation of alternate pathways such as interferon type I or IL-1β have been advanced, together with a potential favoring action on occult infection, which is a well-known trigger for HS.10 To the best of our knowledge, we have described the possible HS paradoxical onset of HS under anti–IL-17 therapy, suggesting that paradoxical adverse events are not restricted to anti–TNF-α agents and close surveillance of new available biological drugs is warranted to detect the occurrence of new or as yet undescribed events.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Maarouf M., Lee D.E., Shi V.Y. Targeted treatments for hidradenitis suppurativa: a review of the current literature and ongoing clinical trials. J Dermatol Treat. 2017;10:1–9. doi: 10.1080/09546634.2017.1395806. [DOI] [PubMed] [Google Scholar]
- 2.Matusiak Ł., Szczęch J., Bieniek A., Nowicka-Suszko D., Szepietowski J.C. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: implications for treatment with anti-IL-17 agents. J Am Acad Dermatol. 2017;76(4):670–675. doi: 10.1016/j.jaad.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 3.Lima A.L., Karl I., Giner Y.T. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitissuppurativa. Br J Dermatol. 2016;174(3):514–521. doi: 10.1111/bjd.14214. [DOI] [PubMed] [Google Scholar]
- 4.Thorlacius L., Theut Riis P., Jemec G.B.E. Severe hidradenitis suppurativa responding to treatment with secukinumab: a case report. Br J Dermatol. 2017;179(1):182–185. doi: 10.1111/bjd.15769. [DOI] [PubMed] [Google Scholar]
- 5.Schuch A., Fischer T., Boehner A., Biedermann T., Volz T. Successful treatment of severe recalcitrant hidradenitis suppurativa with the interleukin-17A antibody secukinumab. Acta Derm Venereol. 2017;98(1):151–152. doi: 10.2340/00015555-2794. [DOI] [PubMed] [Google Scholar]
- 6.RavJørgensen Astrid-Helene, Yao Yiqiu, Francis Thomsen Simon. Therapeutic response to secukinumab in a 36-year-old woman with hidradenitis suppurativa. Case Rep Dermatol Med. 2018;2018 doi: 10.1155/2018/8685136. 8685136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giuseppe Pistone, Pardo Nicola, Valentina Caputo. A case of moderate hidradenitis suppurativa and psoriasistreated with secukinumab. Ann Dermatol. 2018;30(4):462–464. doi: 10.5021/ad.2018.30.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toussirot É., Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;15(2):e000239. doi: 10.1136/rmdopen-2015-000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faivre C., Villani A.P., Aupin F. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74(6):1153. doi: 10.1016/j.jaad.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Tillack C., Ehmann L.M., Friedrich M. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL- 17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63:567–577. doi: 10.1136/gutjnl-2012-302853. [DOI] [PubMed] [Google Scholar]