Abstract
Crossed cerebellar diaschisis (CCD) represents the reduction of blood flow, metabolism, and oxygen consumption in the cerebellar hemisphere contralateral to a cerebral focal lesion. This phenomenon is the result of remote metabolic effects of cerebral lesions and it has been described since the first attempts for functional imaging of the brain, almost 40 years ago. Nevertheless, its clinical significance remains uncertain and new ways to use imaging of CCD for prognosis or assessment of novel therapies are being investigated. In this report, we present treatment for glioblastoma as a cause of CCD imaged on positron emission tomography/computed tomography with (18F) fluoro-D-glucose in our department.
Keywords: Crossed cerebellar diaschisis, fluoro-D-glucose, glioblastoma, positron emission tomography/computed tomography, stroke
INTRODUCTION
Crossed cerebellar diaschisis (CCD) is defined as the reduction of blood flow, metabolism, and oxygen consumption in the cerebellar hemisphere contralateral to a supratentorial focal lesion.[1,2] This phenomenon was discovered and described during the first attempts for functional imaging of the brain,[3] and although it has been demonstrated in animals and humans with various types of lesions, its clinical significance remains uncertain.[4] CCD has been reported on positron emission tomography/computed tomography (PET/CT) studies with (18F) fluoro-D-glucose (FDG) for the assessment of brain lesions or as an incidental finding. In this report, we present a case of CCD imaged on follow-up PET/CT with (18F) FDG in a patient who had received treatment for glioblastoma multiforme.
CASE REPORT
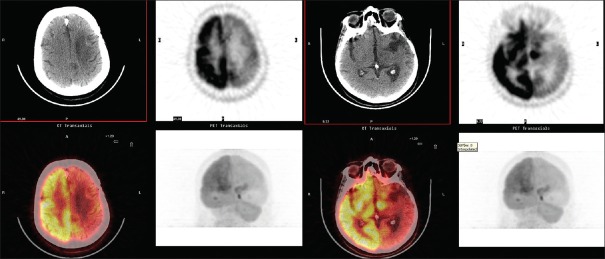
A 53-year-old woman with a history of glioblastoma multiforme in the left basal ganglia was referred to our department for PET/CT with (18F) FDG. The patient had been initially treated with surgery and radiation. Two years later, recurrence of the tumor had occurred and she had been treated with γ-knife and a chemotherapy regimen. One month after the end of treatment, the patient was referred for PET/CT for the evaluation of response. The uptake of (18F) FDG was diffusely decreased in the left cerebral hemisphere and also in the right cerebellar hemisphere [Figure 1], which is the characteristic image for CCD.
Figure 1.
Crossed cerebellar diaschisis in positron emission tomography/computed tomography with (18F) fluoro-D-glucose in a 53-year-old woman with a history of glioblastoma multiforme in the left temporal lobe, after treatment for recurrence. The patient had initially been treated with surgery and radiation and, 2 years later, with γ-knife and chemotherapy for recurrence. The uptake of (18F) fluoro-D-glucose is diffusely decreased in the left cerebral hemisphere and in the contralateral cerebellar hemisphere
DISCUSSION
CCD is one of the first reported and most studied phenomena of remote metabolic depression, which result from the disruption of synaptic networks by focal brain lesions.[4,5] In CCD, the affected pathway is the glutamatergic crossed corticopontocerebellar descending pathway, which is predominantly excitatory.[5]
CCD was imaged for the first time in 1981 by Baron et al. in a patient with stroke who underwent PET with inhaled 15O2 for quantification of cerebral blood flow and oxygenation.[3] Since then, the concept of CCD has been closely associated with the attempts of nuclear medicine for functional imaging of blood flow, oxygenation, and metabolism of the brain with various modalities and radiopharmaceuticals, such as PET with 15O2, (15O) H2O, (18F) FDG or SPECT with (99mTc) Tc-HMPAO, (99mTc) Tc-ECD, (123I) IMP, and for quantification of these parameters with various indices, such as regional cerebral blood flow and regional cerebral glucose metabolism.[3] Irrespective of the radiotracer, the modality, and the targeted mechanism, the characteristic imaging pattern of CCD consists of diffusely decreased uptake in the affected cerebral hemisphere and in the contralateral cerebellar hemisphere.
The wide use of PET/CT with (18F) FDG for functional imaging of metabolism is a result of the incidental imaging of anatomically and clinically occult CCD.[6] The most common cause of CCD is stroke and it is prevalent in approximately 50% of patients with cortical or subcortical strokes.[5] CCD has been almost exclusively studied in patients with stroke and the phenomenon's topography and time course have been correlated with the respective stroke's features.[2,5] Nevertheless, specific clinical correlations of CCD have not been proven and its significance as a prognostic marker for the outcome of ischemic strokes has not been elucidated. There are also reports of other causes of CCD, such as head injury, encephalitis, epilepsy,[7] and Creutzfeld–Jacob disease.[8]
CCD in PET/CT with (18F) FDG in patients with primary and metastatic tumors of the brain, including glioblastoma, as in the patient presented in this study, has been sporadically reported.[9,10] Although the patients in these studies had also received treatment such as resection, radiation, and/or chemotherapy, the appearance of CCD was not specifically correlated with any of these interventions; it was rather used as a prognostic marker for the overall survival or to highlight the significance of metabolic characterization with (18F) FDG PET/CT, especially when complemented with other radiotracers, such as (18F) fluoro-L-dihydroxyphenylalanine or modalities, such as PET/magnetic resonance imaging.[7] It has also been hypothesized that the appearance of CCD, particularly when accompanied by clinical symptoms, might be a sign of occult recurrence with widespread invasive disease.[10]
CONCLUSION
Diaschisis is a 100-year-old concept that describes remote metabolic effects of focal lesions of the brain. The clinical significance of these effects has not yet been documented. Nevertheless, neuroscientists are investigating new ways to use the ability to image and quantify these effects with the help of nuclear medicine, either for prognosis or for the assessment of noninvasive neuromodulation techniques as potential therapeutic options for stroke and other brain lesions.[4,10]
This case report adds to the limited literature on the appearance of CCD in follow-up PET/CT examinations with (18F) FDG in patients after treatment for glioblastoma and highlights the need for further investigation of the phenomenon through nuclear medicine imaging methods, in order to establish its clinical utility.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Komaba Y, Mishina M, Utsumi K, Katayama Y, Kobayashi S, Mori O, et al. Crossed cerebellar diaschisis in patients with cortical infarction: Logistic regression analysis to control for confounding effects. Stroke. 2004;35:472–6. doi: 10.1161/01.STR.0000109771.56160.F5. [DOI] [PubMed] [Google Scholar]
- 2.Pantano P, Baron JC, Samson Y, Bousser MG, Derouesne C, Comar D, et al. Crossed cerebellar diaschisis. Further studies. Brain. 1986;109(Pt 4):677–94. doi: 10.1093/brain/109.4.677. [DOI] [PubMed] [Google Scholar]
- 3.Baron JC, Bousser MG, Comar D, Castaigne P. “Crossed cerebellar diaschisis” in human supratentorial brain infarction. Trans Am Neurol Assoc. 1981;105:459–61. [PubMed] [Google Scholar]
- 4.Carrera E, Tononi G. Diaschisis: Past, present, future. Brain. 2014;137:2408–22. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- 5.Lewis DH, Toney LK, Baron JC. Nuclear medicine in cerebrovascular disease. Semin Nucl Med. 2012;42:387–405. doi: 10.1053/j.semnuclmed.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Garg G, Tripathi M, D’ Souza MM, Sharma R. Crossed cerebellar diaschisis demonstrated by (18) F- FDG-PET/CT. Hell J Nucl Med. 2009;12:171–2. [PubMed] [Google Scholar]
- 7.Han S, Wang X, Xu K, Hu C. Crossed cerebellar diaschisis: Three case reports imaging using a tri-modality PET/CT-MR system. Medicine (Baltimore) 2016;95:e2526. doi: 10.1097/MD.0000000000002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kastenbauer S, Schulz-Schaeffer WJ, Tatsch K, Yousry TA, Kretzschmar HA, Pfister HW, et al. Crossed cerebellar diaschisis: A clue to the mechanism of ataxic hemiparesis in Creutzfeldt-Jakob disease? J Neurol. 2001;248:1093–5. doi: 10.1007/pl00007828. [DOI] [PubMed] [Google Scholar]
- 9.Calabria F, Schillaci O. Recurrent glioma and crossed cerebellar diaschisis in a patient examined with 18F-DOPA and 18F-FDG PET/CT. Clin Nucl Med. 2012;37:878–9. doi: 10.1097/RLU.0b013e318262af2a. [DOI] [PubMed] [Google Scholar]
- 10.Segtnan EA, Grupe P, Jarden JO, Gerke O, Ivanidze J, Christlieb SB, et al. Prognostic implications of total hemispheric glucose metabolism ratio in cerebrocerebellar diaschisis. J Nucl Med. 2017;58:768–73. doi: 10.2967/jnumed.116.180398. [DOI] [PubMed] [Google Scholar]