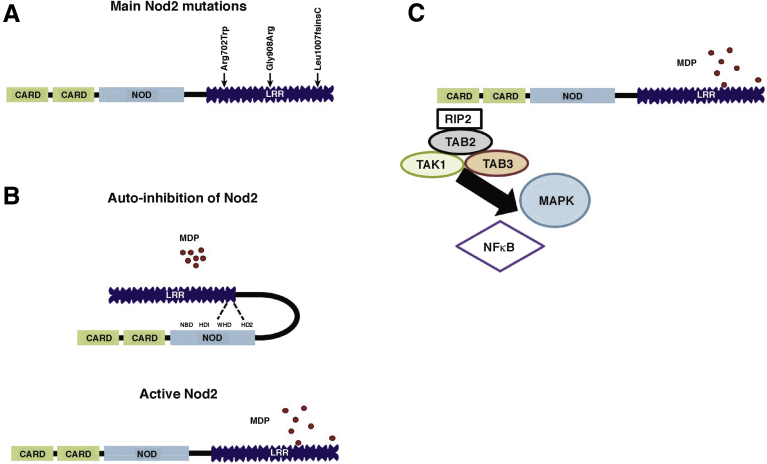
Figure 1.
Structure and main intracellular pathway induced by NOD2. (A) NOD2 protein shows 3 domains including CARDs, NOD, and leucine-rich repeats (LRRs). Within the LRR region, ↓ indicates an amino acid change owing to a CD-associated polymorphism. (B) The NOD module contains a nucleotide-binding domain (NBD), a winged helix (WH), and 2 helix domains (HD1 and HD2). The interaction between NBD and WH is important to stabilize Nod2 in an inactive form, and is maintained by adenosine diphosphate-mediated packed conformation. Upon ligand binding, HD2 mediates conformational changes of the NBD, WH, and HD1 to allow adenosine diphosphate–adenosine triphosphate exchange, self-oligomerization, and downstream signaling. The effector CARD domains mediate intracellular signaling after interaction between the LRR domain and MDP. (C) NOD2 oligomerization induces a signaling complex named nodosome. NOD2 attracts receptor-interacting serine/threonine-protein kinase 2 (RIP2) via a CARD–CARD homotypic interaction followed by transforming growth factor β-activated kinase 1 (TAK1) and TAK1 binding protein 2 and 3 (TAB2 or 3). This complex induces the activation of both MAPKs and NF-κB pathways.