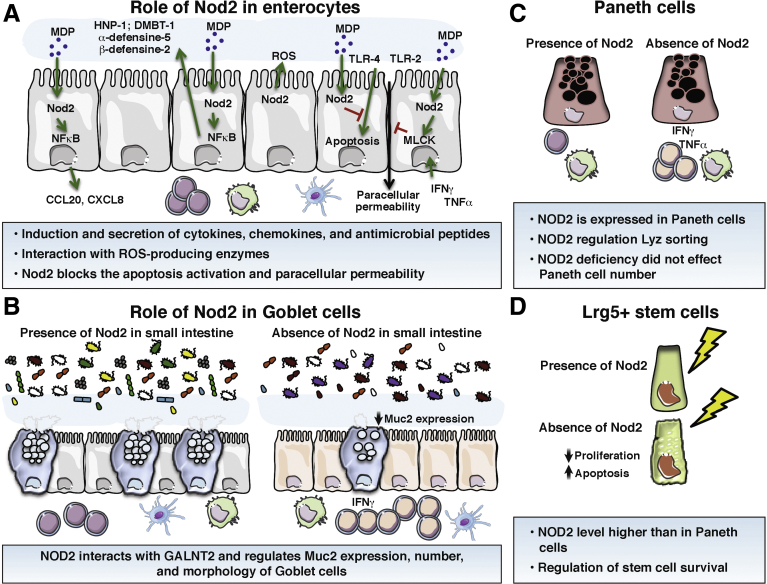
Figure 2.
Role of Nod2 in the homeostasis of the main cell types of the intestinal epithelium. (A) Enterocytes. Intestinal enterocyte cells express NOD2 and its expression is up-regulated by inflammatory cytokines. In response to MDP, intestinal epithelial cells synthesize AMPs, controlling the growth of pathogenic bacteria. This AMP secretion is lost in case of NOD2 mutation or deficiency. MDP stimulation also is able to induce the secretion of CXCL-8 to recruit neutrophil cells. Nod2 stimulation blocks the apoptosis induced by TLR-4 and the increased paracellular permeability induced by TLR-2, TLR-4, or by TNF-α and IFN-γ. (B) Goblet cells. A reduced mucin 2 expression as well as a diminished number of goblet cells have been described in the small intestine of Nod2KO mice. Nod2KO mice also showed fewer mucin granules per goblet cell than in WT mice. (C) Paneth cells. They express NOD2, and NOD2 expression is up-regulated in Paneth cells of CD patients. In these cells, NOD2 activation by MDP enhances the expression of α-human defensin-5 and -6 through induction of the NF-κB pathway. (D) Intestinal stem cells. In the gut, Lgr5+ stem cells strongly expressed Nod2. NOD2 stimulation by MDP promotes colonic epithelial cell growth and protection against apoptosis. NOD2 depletion results in a reduced ability to grow cells and increased levels of apoptosis by conferring cytoprotection against oxidative stress–mediated cell death. CCL20, Chemokine (C-C motif) ligand 20; CXCL-8, C-X-C motif chemokine ligand 8; DMBT-1, deleted in malignant brain tumors 1; GALNT2, Polypeptide N-Acetylgalactosaminyltransferase 2; HNP-1, Human Neutrophil peptide-1; Lyz, lysosome; ROS, reactive oxygen species.