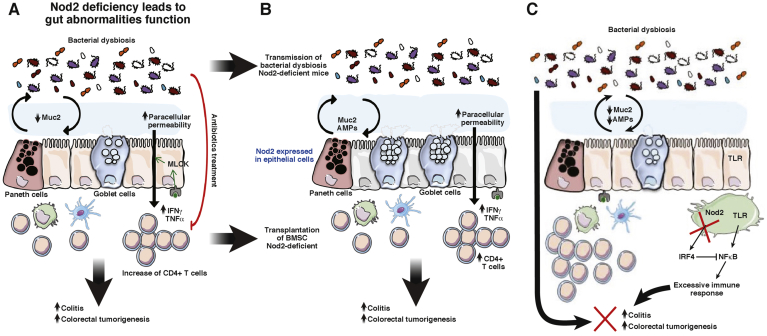
Figure 3.
Mechanisms by which Nod2, in epithelial cells, participates in the development of inflammation and inflammatory-associated carcinogenesis. (A) Nod2 deficiency or mutations have been described to alter gut epithelial permeability, AMP, and mucin expression; to increase CD4 T cells expressing proinflammatory cytokines; and to induce a microbial dysbiosis favoring the development of inflammation and CRC. Antibiotics suppressed the excessive permeability and the alteration of the immune compartment of Nod2KO mice. (B) Transfer of gut microbiota dysbiosis linked to Nod2 deficiency to WT mice alters the expression of both AMPs and mucins secreted by epithelial cells and increases colitis and colorectal cancer susceptibility. Transfer of bone marrow stem cells show that Nod2 expression in the hematopoietic compartment increases CD4 T cells and regulates gut epithelial permeability. However, bone marrow transfer does not change the gut microbiota, showing that Nod2 expression in the immune system plays a pivotal role in the regulation of colitis, independently of microbiota composition. (C) This notion was highlighted recently by the study by Udden et al,77 reporting the development of colonic inflammation and linked colorectal carcinogenesis in Nod2KO mice independently of gut microbiota dysbiosis but resulting from an absence of TLR down-regulation by Nod2.