Figure 5.
acDC Stimulation in G-Rex Devices Further Increases the Yield of Ag-Specific CD8+ T Cells
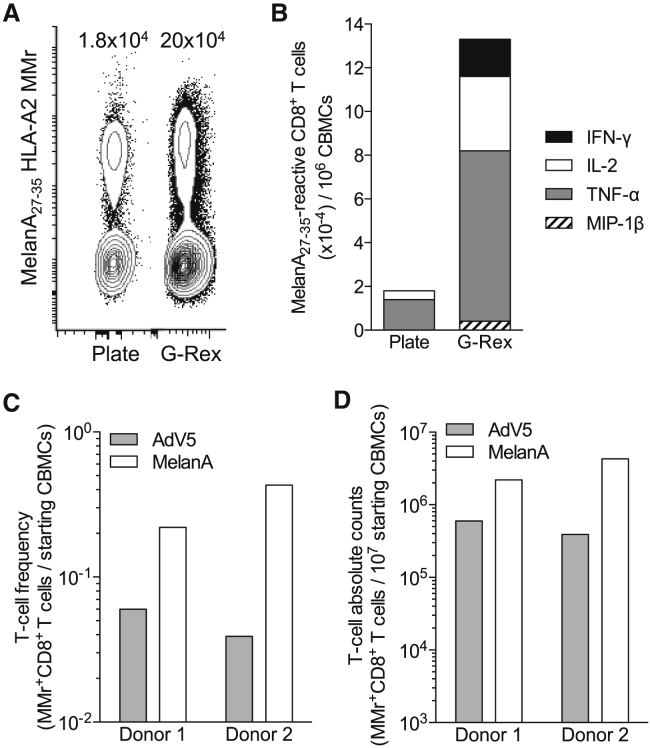
(A) CBMCs were cultured in 12-well plates (10 × 106 CBMCs per well) or G-Rex10 flasks (10 × 106 CBMCs per flask) using a combination of GM-CSF, IL-4, IL-1β, and Flt3L, followed by TLR8L, TNF-α, PGE2, and low-dose IL-7 along with MelanA26–35 peptide after 24 h and by IL-2, IL-15, and IL-7 from day 2 of culture. The number of MelanA26–35-specific CD8+ T cells obtained at the end of the 10-day culture is represented after gating on viable CD8+ T cells and was counted using MelanA26–35-loaded HLA-A2 MMrs. The absolute numbers of MMr+ cells per million CBMCs obtained are indicated, as determined with CountBright beads. (B) The same culture was tested at day 13 during a 6-h recall assay in the presence of LCL cells pulsed with MelanA26–35 or no peptide. The graph displays absolute numbers of CD8+ cells per million CBMCs producing the indicated cytokines in response to MelanA26–35-pulsed LCL cells after background subtraction, i.e., the number of cytokine-positive CD8+ T cells detected in response to unpulsed LCL cells. (C and D) CBMCs from two representative donors were cultured as described earlier in G-Rex10 flasks (10 × 106 CBMCs per flask) with MelanA26–35 or pooled AdV5 Hexon542–550, Hexon892–901, and Hexon916–925 peptides. The number of peptide-reactive CD8+ T cells obtained at the end of the 10-day culture was analyzed after gating on viable CD8+ T cells using HLA-A2 MMrs loaded with the corresponding peptides. (C) The frequency of MMr+ cells out of total CBMCs. (D) The absolute numbers of MMr+ cells obtained from the same cultures, as determined with CountBright beads.