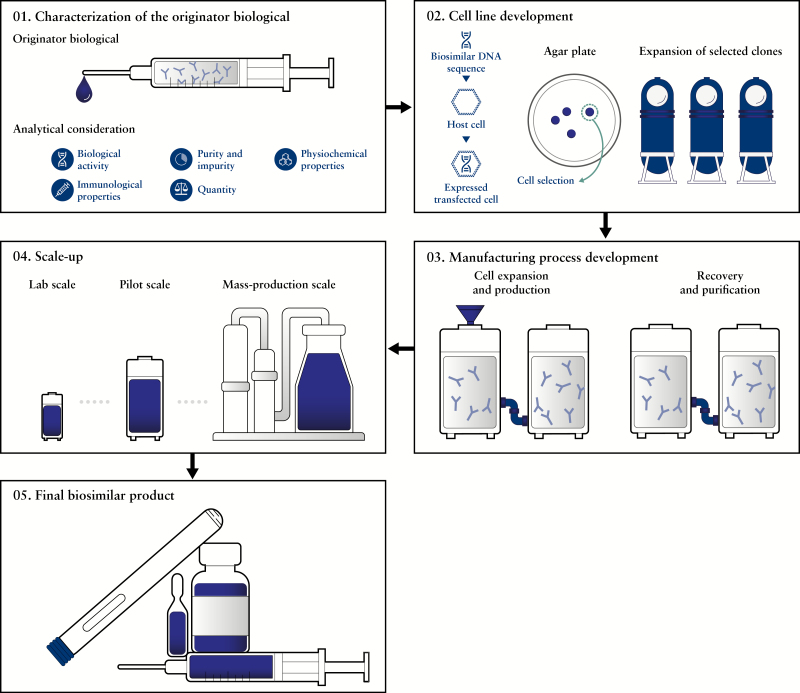
Figure 1.
Development process of biosimilarsa. aFigure adapted from ‘Science behind biosimilars’, Samsung Bioepis Available at: http://www.samsungbioepis.com/file/Science_of_Biosimilars.pdf [Accessed May 2018] 01. The originator biologic first undergoes analyatical consideration to identify its quality attritubes. The data collected are then used to define the critical quality attributes (CQAs) of a biosimilar. 02. The DNA of a biosimilar is incorporated into an expression vector, which is transfected into a host cell to produce a stable expression of the biosimilar. Greater than 15 000 single clones are typically screened to generate the lead cell line that will be the production source of the biosimilar. 03. The lead cell line of a biosimilar is manufactured at lab scale, and the cell culture conditions are refined to increase yield, reduce impurities and maintain optimal growth conditions as a means of achieving consistency and quality. 04. Pilot studies are conducted to maintain real-time monitoring of biosimilar quality control from lab scale to mass-production scale and ensure that the essential quality attributes of the biosimilar are maintained. 05. Final biosimilar products are manufactured under cGMP regulations and must meet the requirements of various regulatory agencies. The final form of biosimilars can be a vial, a pre-filled syringe or an auto-injector. cGMP, current Good Manufacturing Practice; CQA, Critical Quality Attributes; DNA, deoxyribonucleic acid