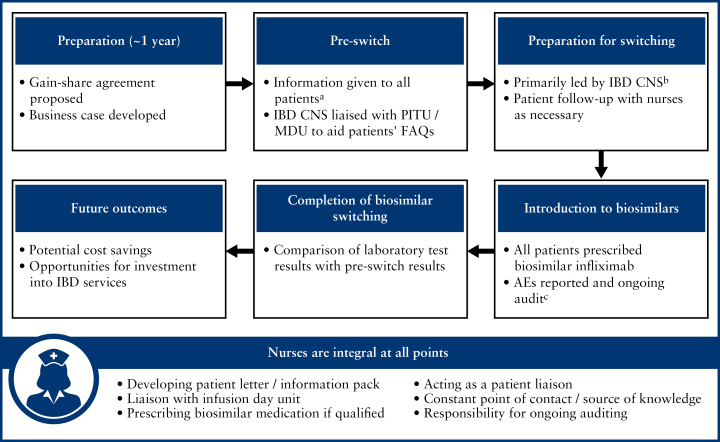
Figure 2.
Biosimilar managed switching programme at the Royal Free London NHS Foundation Trust, UK.47a Letters and information pack posted to patients or daycare nurses distributed information packs at time of patient biologic infusion. All patients had the opportunity to telephone an IBD CNS with questions or arrange a face-to-face conversation at the time of their treatment infusion; patients with further concerns were scheduled to visit their IBD consultant by an IBD CNS or administrator bPreparation for switching was primarily led by the IBD CNS; all patients received the FAQ leaflet, detailed information of the timeline of events, and the planned start date of switching cThe pharmacy and IBD CNS documented the patients switching to biosimilar infliximab and all adverse reactions or prescribing errors were logged. A weekly report was given on each patient at the biologics MDT AE, adverse events; CNS, clinical nurse specialists; FAQs, frequently asked questions; IBD, irritable bowel disease; MDU, Medical Day Unit; MDT, multidisciplinary team; PITU, Planned Investigation Treatment Unit