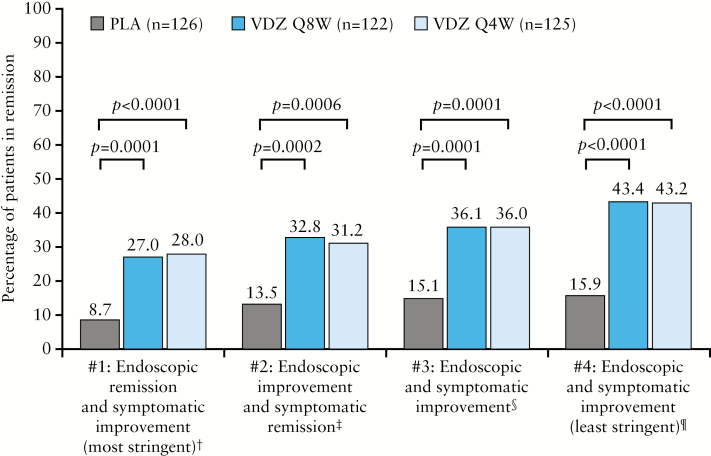
Figure 1.
Deep remission at Week 52a,b [maintenance ITT population]. One patient randomized to PLA still had active drug concentrations [9 μg/mL] at Week 46 and they were in remission according to all four definitions at Week 52. ES, endoscopic score; ITT, intention to treat; PLA, placebo; Q4W, every 4 weeks; Q8W, every 8 weeks; RBS, rectal bleeding score; SFS, stool frequency score; VDZ, vedolizumab. aPost hoc analyses; ball patients in the maintenance ITT population, including those in the PLA group, had received VDZ at Weeks 0 and 2 and had a clinical response at Week 6; †definition 1: Mayo Clinic ES = 0, RBS = 0 and decrease in SFS or no change from baseline [A]; ‡definition 2: Mayo Clinic ES ≤ 1, RBS = 0 and SFS = 0 [B]; §definition 3: Mayo Clinic ES ≤ 1, RBS = 0, decrease in SFS or no change from baseline, and total score [ES + RBS + SFS] ≤ 1 [C]; ¶definition 4: Mayo Clinic ES ≤ 1, RBS = 0 and SFS ≤ 1 [D].