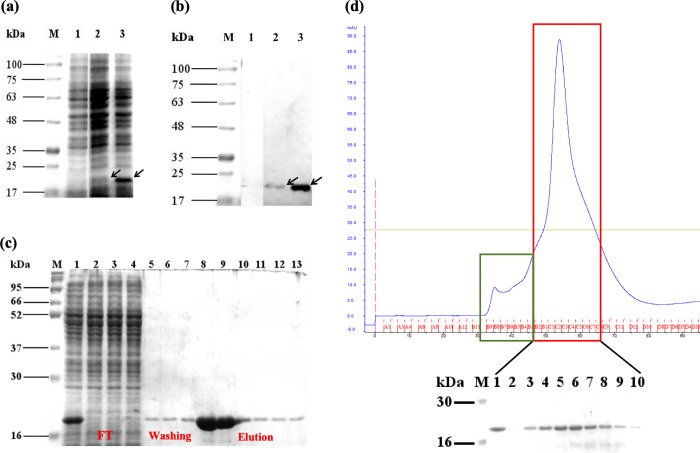
Fig 2. SDS-PAGE and western blot analyses of the recombinant protruding (P) domain of Macrobrachium rosenbergii nodavirus capsid protein (MrNV-CP) expressed in Escherichia coli.
(a) SDS-PAGE, and (b) western blot analysis of the P-domain of MrNV-CP after isopropyl β-D-1-thiogalactopyranoside (IPTG) induction at 30°C. Lane M: protein markers in kDa; lane 1: cell lysate before IPTG induction; lane 2: cell lysate wihout IPTG indution; lane 3: cell lysate after being induced with IPTG for 2 hours. (b) the blot was probed with anti-His monoclonal antibody. Arrowheads indicate additional protein band ~18 kDa in both IPTG induced and uninduced cells. (c) immobilized metal ion affinity chromatography (IMAC) purification. Lane M: protein markers in kDa; lane 1: crude lysate; lanes 2 and 3: flow through; lanes 4–7: fractionated protein samples washed out with washing buffer; lanes 8–13: fractionated protein samples eluted out with elution buffer. (d) Gel filtration chromatogram of the P-domain of MrNV-CP fractionated by HiPrep 16/60 Sephacryl S-200 HR on an FPLC system. The first peak (green box) comprises of elution volume between 33 ml and 45 ml while the second peak (red box) comprises of elution volume between 45 ml and 63 ml. SDS-PAGE analysis of the eluted fractions of the second peak is shown under the chromatogram. Lane M: protein markers in kDa; lane 1: injected sample; lanes 2–10: eluted fractions of the second peak.