Abstract
HSP90 inhibitors have been shown to kill Epstein-Barr virus (EBV)-infected cells by reducing the level of EBV EBNA-1 and/or LMP1. We treated virus-infected cells with ganetespib, an HSP90 inhibitor currently being evaluated in multiple clinical trials for cancer, and found that the drug killed EBV-positive B and T cells and reduced the level of both EBV EBNA-1 and LMP1. Treatment of cells with ganetespib also reduced the level of pAkt. Ganetespib delayed the onset of EBV-positive lymphomas and prolonged survival in SCID mice inoculated with one EBV-transformed B cell line, but not another B cell line. The former cell line showed lower levels of EBNA-1 after treatment with ganetespib in vitro. Treatment of a patient with T cell chronic active EBV with ganetespib reduced the percentage of EBV-positive cells in the peripheral blood. These data indicate that HSP90 inhibitors may have a role in the therapy of certain EBV-associated diseases.
Keywords: ganetespib, HSP90, Epstein-Barr virus, Burkitt lymphoma
Introduction
Epstein-Barr virus (EBV) is associated with several malignancies including Hodgkin lymphoma, Burkitt lymphoma, non-Hodgkin lymphoma, T cell lymphoma, nasopharyngeal carcinoma, and gastric carcinoma [1]. While cytotoxic chemotherapy, radiation, and surgery are effective for many patients with these diseases, others suffer relapses, have long-term side effects associated with chemotherapy, or develop secondary malignancies. Thus, there is a need for additional therapies.
Heat shock proteins are molecular chaperones that are important for protein folding after translation and reduce protein aggregation during cellular stress [2]. Heat shock proteins are thought to be particularly important to stabilize oncoproteins that are frequently mutated or expressed at high levels in tumor cells often located in low oxygen and acidic environments. Heat shock proteins are also important for growth of viruses that rapidly produce large quantities of foreign proteins in the cell [3, 4].
Heat shock protein 90 (HSP90) and HSP70 are induced in B cells early during EBV infection [5]. HSP90, but not HSP70, is expressed on the surface of EBV-transformed B cells [6] HSP90 is upregulated in biopsies from patients with EBV-positive post-transplant lymphoproliferative disease [7].
EBV EBNA-1 along with LMP1 and LMP2 are expressed in EBV-transformed B cells and EBV-positive Hodgkin lymphoma, T cell lymphoma, nasopharyngeal carcinoma, and many non-Hodgkin lymphomas [1]. Inhibition of HSP90 with 17-DMAG, 17-AAG, or geldanamycin reduced expression of EBNA-1, but not LMP1 in EBV-transformed B cells and EBV-positive Burkitt lymphoma cells in vitro [8]. 17-DMAG killed EBV-transformed B cells in vitro and 17-AAG inhibited tumor formation when the transformed B cells were implanted into SCID mice. In contrast, 17-AAG reduced expression of LMP1, but not EBNA-1, in EBV-positive NK cells [9]. 17-AAG also killed EBV-positive NK cells in vitro and inhibited growth of tumors when the cells were inoculated into NOG mice. Another HSP90 inhibitor, BIIBO21 inhibited expression of both EBNA-1 and LMP1 in EBV-positive T and NK cells, decreased the viability of the cells, and inhibited the growth of EBV-positive NK cells after implantation in NOG mice [10]. Geldanamycin also killed EBV-positive NK-T lymphoma cells [11] and another HSP90 inhibitor, AT13387, reduced tumor formation in nude mice injected with EBV-positive nasopharyngeal carcinoma cells [12].
HSP90 inhibitors also can inhibit expression of other viral and cellular proteins in virus-infected cells. 17-DMAG reduced the expression of several herpesvirus protein kinases, including EBV BGLF4, cytomegalovirus UL97, and Kaposi’s sarcoma associated herpesvirus (KSHV) protein kinase [13]. Geldanamycin inhibited expression of cdc2 and Akt, and reduced phosphorylation of Akt in EBV-infected B and NK-T cells [11, 13]. Another HSP90 inhibitor, AT13387, reduced expression of Akt, pAkt, EGFR, CDK2, CDK4, and Skp2 in EBV-positive nasopharyngeal carcinoma cells [13]. Treatment of KSHV-positive primary effusion lymphoma cells with another HSP90 inhibitor, PU-H71, showed that the inhibitor interacted with numerous cellular proteins important for NF-κB activation, apoptosis, autophagy, Akt activation, IL-6 signaling, and angiogenesis [14].
Here we tested an HSP90 inhibitor, ganetespib, currently in multiple clinical trials, for its ability to kill EBV-positive B and T cells, its effect on expression of viral and cellular proteins, and its ability to reduce EBV-infected cells in a patient with chronic active EBV disease.
Methods and Materials
Cell Lines and Reagents
EBV-transformed lymphoblastoid cell lines (LCLa and LCLb), EBV-positive Burkitt lymphoma (BL) cell lines (Akata [15], Kem I and Mutu I [16]), EBV-negative Burkitt lymphoma cell lines (BJAB [17] and BL30 [18]), and an EBV-positive T cell line (SNT-16 [19, 20]) were studied. All cells lines were propagated in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin, and streptomycin. The medium for the SNT-16 cell line was also supplemented with 600 units/mL IL-2 (NCI Biological Resources Branch, Frederick, MD). Ganetespib was provided by Synta Pharmaceutical Corp. (Lexington, MA) and was dissolved in dimethyl sulfoxide (DMSO) as a 10 mM stock.
Cell Viability Assay
Cells (4-6 × 104 cells) were cultured in flat bottom wells of a 96-well plate at 37°C in RPMI 1640 medium with 10% FBS in the presence of ganetespib for three days. To measure viability, cells were incubated for 2 hr at 37°C with a 1:1 mixture of alamarBlue (Invitrogen, Carlsbad, CA) and RPMI 1640 medium with 10% FBS. Proliferating cells reduce alamarBlue to a fluorescent compound, which is quantified using a Syngeny 2 multiwell plate reader (BioTek, Winooski, VT) with excitation of 535 nm and emission of 595 nm. Trypan blue exclusion was used to measure viability of peripheral blood mononuclear cells (PBMCs), since these cells do not proliferate sufficiently in the absence of stimuli to reduce alamarBlue.
Immunoblots
Cells treated with ganetespib were lysed in RIPA buffer containing 10 mM Tris-HCl pH 8, 100 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholate, 0.5% SDS, and a cocktail of protease inhibitors (Roche, Indianapolis, IN). Equal amounts of protein were fractionated on SDS-PAGE gels, transferred to nitrocellulose membranes, and probed with various antibodies. After washing, the membranes were incubated with anti-mouse or anti-rabbit antibodies conjugated to HRP, washed, and developed using enhanced chemiluminescence. The primary antibodies used were mouse anti-EBNA-1 (Fitzgerald Industries International, Acton, MA), mouse anti-LMP1 (DAKO, Carpinteria, CA), mouse anti-BZLF1 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-HSP70 (Enzo Life Sciences, Farmingdale, NY), rabbit anti-Akt and rabbit pAkt (S473) (Cell Signaling, Beverly, MA), and mouse anti-β-actin (Sigma-Aldrich, St. Louis, MO) antibodies. For cell sorting, mouse anti-Vβ5.1 FITC (Beckman-Coulter) and CD4-PE (BioLegend) antibodies were used.
Animal Experiments
Female SCID mice, aged 4–6 weeks, were inoculated intraperitoneally with 1 × 106 LCLs and 7 days after inoculation began treatment with ganetespib. Animals were treated with 150 mg/kg of ganetespib in DMSO diluted in a solution of 18% Cremophor® RH40 (Polyoxyl 40 hydrogenated castor oil) and 3.6% dextrose or DMSO and vehicle control by tail vein injection one day a week for 6 weeks. Mice were observed daily for survival and were autopsied to detect ascites and solid tumors. All mouse experiments were performed under a protocol approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases.
Treatment of patient with chronic active EBV infection
A 67-year-old man with T cell chronic active EBV and hydroa vacciniforme (previously reported in [21]) signed an informed consent on a study protocol approved by the Institutional Review Board of the National Institute for Allergy and Infectious Diseases. He was treated with ganetespib intravenously with the drug given in cycles of treatment for two consecutive weeks followed by no therapy for one week, in escalating doses from 60 to 150 mg/M2 for a total of 8 cycles.
Fluorescence in situ hybridization
EBV genomes were detected in peripheral blood cells as previously described [22]. Briefly, cells were incubated for 15 min with a solution of 0.2N acetic acid, 0.02N HCl, in Tris HCl, placed onto glass microscope slides, fixed with BS3 (Thermo Fisher Scientific), permeabilized with Triton X-100, washed with PBS and incubated in RNAse T1 and RNAse A. The cells were then incubated in 0.1M Tris-HCl/0.1M glycine, washed in 2× SSC, incubated in hybridization buffer (Enzo Life Sciences), denatured at 94°C, hybridized to a biotinylated EBV BioProbe (Enzo Life Sciences) for 6 min at 85°C, and then hybridized overnight at 37°C. After extensive washing, the cells were incubated with Alexa 594-conjugated streptavidin, and visualized with a confocal microscope. Photographs of serial z-stack sections were obtained throughout the cells and then overlapped to quantify the total number of EBV genomes (each genome corresponds to one fluorescent spot).
Results
EBV-positive and EBV-negative B cell lines are equally susceptible to killing by ganetespib
EBV-positive and EBV-negative B cell lines were treated with various concentrations of ganetespib for 72 hr. Two of the EBV-positive Burkitt lymphoma cells which have a type 1 latency pattern (Akata, Mutu I), both of the EBV-negative Burkitt lymphoma cell lines (BJAB and BL30), and both of the EBV-transformed B cells (LCLa and LCLb) were slightly more sensitive than the third EBV-positive Burkitt lymphoma cell line (Kem I). All cells except for the Kem I cells showed greater than 70% loss of cell viability at a dosage of 37 nM ganetespib (Fig. 1A). At the same concentration of ganetespib, Kem I cells showed approximately 50% loss of cell viability. The average effective concentration at which 50% of the cells were killed (EC50) ranged from 19.5 nM to 22.5 nM ganetespib for the two EBV-transformed LCLs, while the EBV-negative cell lines (BJAB and BL30) had an EC50 of 25.5 nM (Table I). The EBV-positive Burkitt lymphoma cells with a type 1 latency pattern (Akata, Kem I, and Mutu I) had EC50s that ranged from 25.5 nM to 37 nM. One EBV-positive and one EBV-negative T cell line were also treated with different concentrations of ganetespib and the T cells were slightly less sensitive to ganetespib than the B cells (EC50 for EBV-positive SNT16 cells was 41 nM) (Fig. 1B). The toxicity of ganetespib was also examined by treating freshly isolated human PBMCs with various concentrations of ganetespib for three days at which time there was minimum toxicity at concentrations less than 250 nM of ganetespib (Fig. 1C).
Figure 1.
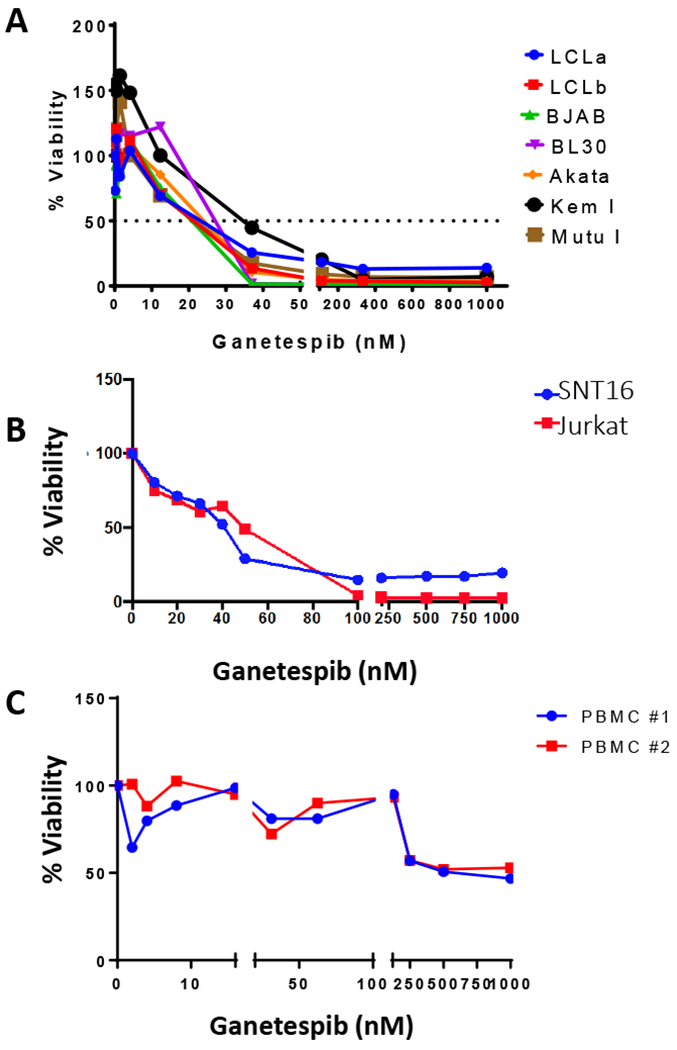
Ganetespib kills EBV-positive and EBV-negative cells lines in a dose-dependent manner. A. EBV-positive (LCLa, LCLb, Akata, Kem I, Mutu I) and EBV-negative (BJAB, BL30) cells were treated with increasing concentrations of ganetespib for 72 hr and cell viability was measured using alamarBlue. A representative experiment is shown in which each point represents the mean of triplicate replicates. B. EBV-positive (SNT16) and EBV-negative (Jurkat) cells were treated as in panel A. The experiment was repeated twice and a representative result is shown. C. Freshly isolated human PBMCs from different donors were treated with increasing concentrations of ganetespib for 72 hr and cell viability was analyzed by trypan blue staining. Trypan blue staining was used to measure viability of PBMCs since the cells did not have sufficient metabolic activity to metabolize alamarBlue. The experiment was repeated twice with a total of 4 different donors and similar results were observed.
Table I.
EC50s for EBV-positive and EBV-negative B cell lines treated with ganetespib.
| EC50 (nM)* | SD | EBV Latency State | |
|---|---|---|---|
| LCLa | 22.5 | 0.50 | 3 |
| LCLb | 19.5 | 1.5 | 3 |
| BJAB | 22.5 | 2.5 | EBV negative |
| BL30 | 25.5 | 1.5 | EBV negative |
| Akata | 25.5 | 1.5 | 1 |
| Kem1 | 37 | 2.0 | 1 |
| Mutu I | 26 | 4.0 | 1 |
| SNT16 | 41.3 | 1.4 | 2 |
| Jurkat | 29.3 | 3.6 | EBV-negative |
EC50 is the effective concentration at which 50% of the cells were killed. Each value for the cells in the first seven rows represents the average of two experiments that were performed in triplicate, and for the last two rows each value is the average of three experiments; SD is the standard deviation of the data.
Ganetespib reduces the level of EBV EBNA-1 and LMP1 in most EBV-positive cells lines, but does not induce lytic gene expression
We tested the effect of ganetespib on EBV protein expression in cell lines with each of the three patterns of EBV latency. In cells that possess a type 1 latency pattern (Akata, Kem I, and Mutu I), EBNA-1 is the only protein expressed. Cells that have a type 2 latency pattern (SNT16), express the EBNA-1, LMP1, and LMP2 proteins. All of the EBV-associated latency proteins are expressed in cells with a type 3 latency pattern (LCLa and LCLb). The cells were treated with ganetespib or DMSO vehicle control for 24 or 48 hr and lysates were prepared and immunoblotted to measure the expression of EBV latency proteins EBNA-1 and LMP1 (Fig. 2A-C). EBNA-1 was reduced in all of the EBV-positive cells lines tested, LMP1 was reduced in most of the cells lines with EBV latency 2 or latency 3 pattern, and levels of β-actin were unchanged. LCLb consistently showed lower levels of EBNA-1, but not LMP1, after treatment with ganetespib.
Figure 2.
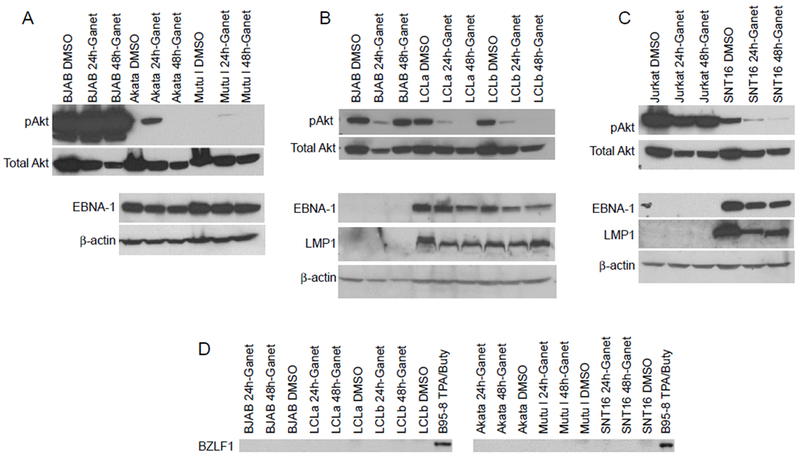
Ganetespib reduces expression of EBNA-1, LMP1, and pAkt in EBV-positive cells. EBV-positive latency type 1 Akata and Mutu I cells (A), latency type 3 LCLa and LCLb (B), latency type 2 SNT16 cells (C), and EBV-negative BJAB cells (A, B) and Jurkat T cells (C) were treated with 50 nM ganetespib (Ganet) or 0.05% DMSO (D) for 24 or 48 hr. Lysates were prepared and analyzed by immunoblotting with anti-EBNA-1, anti-LMP1, pAkt, Akt, and anti-β-actin antibodies. In panel A, top blot, the three lanes for the BJAB cells immunoblotted with antibody to pAkt are from the same blot as the Akata and Mutu I cells incubated with the antibody, but are a lighter exposure. Similar inhibition of EBNA-1, LMP1, and pAkt was seen when the cells were treated with 5 uM ganetespib (data not shown). (D) Cells were treated as in panel A-C and immunoblotted using an anti-BZLF1 antibody. B95–8 cells treated with 12-O-tetradecanoylphorbol 13-acetate (TPA) and sodium butyrate (buty) were used as a positive control for BZLF1.
Lytic replication of EBV is known to induce cell death; therefore, we determined if ganetespib induced virus replication in EBV-positive cells. Cells were treated with ganetespib for 24 or 48 hr and immunoblotted to measure expression EBV BZLF1, a marker of viral lytic replication. Lytic replication was not observed in any cell line tested (Fig. 2D).
HSP90 inhibitors also affect the level of expression of multiple cellular proteins in virus-infected cells, including proteins in the Akt signaling pathway [11, 14]. Ganetespib reduced the level of pAkt in EBV-positive lymphocytes with type 1, 2, or 3 latency (Fig 2A-C).
Ganetespib delays the onset of EBV-positive lymphomas and prolongs survival in SCID mice inoculated with one EBV-transformed B cell line, but not another B cell line
SCID mice injected intraperitoneally with EBV-transformed B cells (LCLs) develop EBV-positive lymphomas that express the same viral latency proteins found in lymphomas from immunocompromised patients [23]. To test the effect of ganetespib in SCID mice, animals were divided into groups of 10 mice and injected with 1 × 106 LCLa or LCLb cells intraperitoneally. Treatment with ganetespib (150 mg/kg) or control (DMSO) was started 7 days post-inoculation of the cells. Animals were treated 1 day a week for 6 weeks. In two independent experiments, animals inoculated with LCLb showed an increase in survival after treatment with ganetespib, compared to those that received the vehicle control (Fig. 3A, B). In these two experiments 80% or 100% of the animals receiving ganetespib survived at 120 days post-inoculation, while only 30% or 50% of the control animals survived for 120 days. Autopsies on animals that died showed lymphomas in all cases. Lymphomas in mice inoculated with EBV LCLs (whether untreated or treated with ganetespib) involved the abdominal wall, and adhered to the mesentery; in some animals, masses are observed that adhered to the outside of the liver and intestine. However, lymphoma was not observed inside the liver or the spleen. In contrast, animals inoculated with LCLa showed no improvement in survival after treatment with ganetespib compared to those that received the vehicle control (Fig. 3C, D). All of the animals that died had lymphomas at autopsy. While animals receiving LCLb and the control vehicle died at a mean of 100 days after inoculation with EBV-transformed B cells, animals receiving LCLa and the control vehicle died a mean of 40 days after receiving these cells. Thus, ganetespib was more effective for the treatment of EBV-transformed B cells that were less malignant in vivo. In addition, as noted above, LCLb had lower levels of EBNA-1, but not LMP1, after treatment with ganetespib than LCLa.
Figure 3.
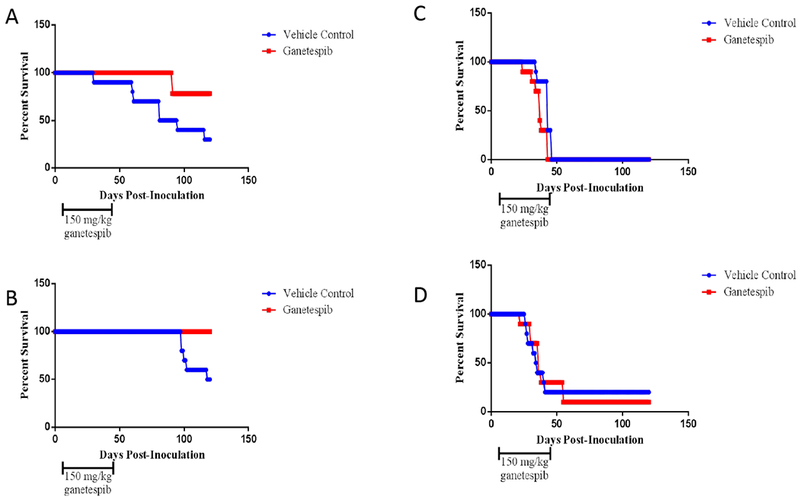
Ganetespib increases survival of SCID mice inoculated intraperitoneally with LCLb, but not LCLa. Groups of 10 animals each were inoculated with 1 × 106 LCLb (A, B) or LCLa (C, D) intraperitoneally and 7 days later treatment with 150 mg/kg ganetespib or vehicle control was started. The animals were treated once a week for 6 weeks and survival of the animals was followed for 120 days. Each experiment was performed in duplicate and shown as A, B or C, D. Comparison of treated and untreated animals showed p=0.031 (A), p=0.011 (B), p=0.018 (C), and p=0.88 (D).
Ganetespib reduces the percentage of EBV-positive cells in the peripheral blood of a patient with chronic active EBV disease
To determine if ganetespib would be effective in humans with EBV disease, we treated a 67-year-old man with T cell chronic active EBV and hydroa vacciniforme who had a very high level of EBV DNA in the peripheral blood (ranging from 100,000 to 5 million copies per ml), with virus-infected T cells in the skin and blood [21]. The patient had a Vβ5.1+CD4+ T cell clone in the peripheral blood that was EBV-positive. The patient had not responded to prior therapy with interferon-α, lenalidomide, hydroxyurea, or vorinostat and ganciclovir.
The patient received 60 mg/M2 of ganetespib intravenously for two consecutive weeks followed by one week with no therapy, then 80 mg/M2 of ganetespib intravenously for two consecutive weeks, followed by one week with no therapy, then 120 mg/M2 of ganetespib intravenously for two consecutive weeks, followed by one week with no therapy, and finally 150 mg/M2 of ganetespib intravenously for two consecutive weeks. The patient tolerated the medication well, with only mild episodes of loose stools (the most common side effect of ganetespib) that were easily controlled with loperamide.
The percentage of EBV-positive cells in the peripheral blood, measured by fluorescence in situ hybridization using an EBV DNA probe, declined by about 50% after treatment with 120 mg/M2 ganetespib and remained at about 50% after treatment with 150 mg/M2 ganetespib (Fig. 4A). In contrast the level of EBV DNA in the blood, measured by PCR, showed no difference during therapy (Fig. 4B).
Figure 4.
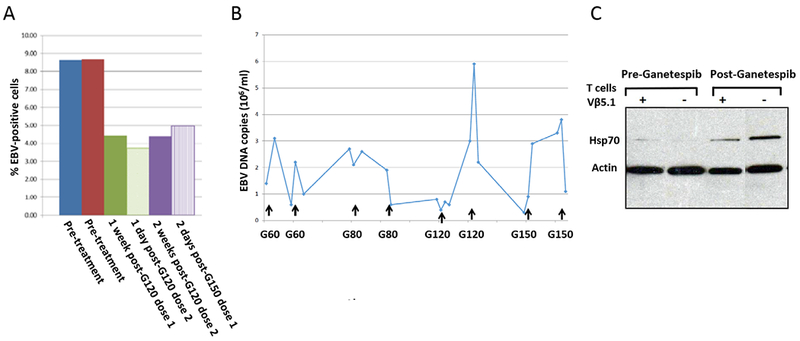
Ganetespib reduces the percentage of EBV-infected cells, but not the level of EBV DNA, in the blood in a patient with hydroa vaccinforme and markedly elevated levels of EBV in the blood. A. PBMCs were isolated from blood, fluorescence in situ hybridization was performed using an EBV DNA probe, and the percentage of EBV infected cells was calculated. B. EBV DNA PCR was performed on peripheral whole blood from the patient before, during, and after ganetespib treatment. The dose of ganetespib (G) is indicated as mg/M2 (e.g. G60 is ganetespib 60 mg/M2). Arrows show doses of the drug. C. Peripheral blood mononuclear cells obtained before, and two days after, receiving 150 mg/M2 of ganetespib were sorted into Vβ5.1+CD4+ (+) and V β5.1−CD4+ (−) populations. Lysates obtained from equivalent numbers of cells from the two populations were run on gels and immunoblotting was performed with antibodies to HSP70 and β-actin.
To verify that ganetespib levels in the blood were sufficient to have a pharmacologic effect on HSPs, we measured the level of HSP70 in the patient’s blood 2 days after receiving gantespib. It has been reported that HSP90 inhibitors reduce the binding of HSP90 to heat shock factor 1, allowing the latter to bind to heat shock elements on the HSP70 gene promoter resulting in increased HSP70 [24]. Treatment of the patient with ganetespib increased the level of HSP70 (Fig. 4C), indicating that ganetespib levels in the blood were affecting HSPs.
Discussion
We found that ganetespib, an HSP90 inhibitor, killed EBV-positive B and T cell lines with various patterns of EBV latent gene expression, reduced levels of EBNA-1 and LMP1 in the cells, and inhibited activation of Akt in vitro. In vivo, ganetespib delayed the time to development of lymphoma in mice inoculated with one, but not another, EBV-transformed B cell line and reduced the percentage of EBV-positive cells in the peripheral blood of a patient with chronic active EBV disease.
Ganetespib is a resorcinol-containing triazole HSP90 inhibitor that is currently in 18 clinical trials including phase 1/2 or phase 2 trials for breast, ovarian, and non-small cell lung cancer, malignant peripheral nerve sheath tumor, mesothelioma, and ocular melanoma as well as a phase 2/3 trial for acute myeloid leukemia [25, 26]. While over 1,300 patients have been treated with the drug, use of ganetespib has not been reported in persons with EBV-related diseases.
Ganetespib showed a similar level of effectiveness for killing EBV-positive and EBV-negative B cells in vitro. In contrast, the level of drug required to kill an EBV-positive T cell line was about two-fold higher than most of the B cell lines. EBV-positive T cell malignancies are generally more difficult to treat than EBV-positive B cell cancers; thus, we tested ganetespib in a patient with chronic active EBV disease with EBV predominantly in T cells.
Ganetespib reduced the levels of both EBV EBNA-1 and LMP1 in EBV-positive B and T cell lines. Prior studies with HSP90 inhibitor 17-AAG showed that it reduced EBNA-1 expression in EBV-positive B cells [8], and LMP1 in EBV-positive T cells [9]. A recent report described another HSP90 inhibitor, B11B021, which reduced the level of both EBNA-1 and LMP1 in EBV-positive B and T cell lines [10]. Thus, ganetespib resembles B11B021 more than 17-AAG, which inhibits either EBNA-1 [8] or LMP1 [9] depending on the cell line tested. Ganetespib reduced the level of activated Akt in EBV-transformed cells and in EBV-negative B and T cell lines and has been shown to reduce Akt activation in other cell types as well [27–29]. Down-regulation of Akt has been postulated to explain the mechanism of cell death caused by geldanamycin in other EBV-infected cells [11].
Treatment of SCID mice with ganetespib, after the animals were inoculated with two different LCLs, showed that ganetespib prolonged survival in mice that received LCLb, but not LCLa. The EC50s for these two cell lines was very similar in vitro (19.5 for LCLb and 22.5 for LCLa); however, LCLb showed consistently increased downregulation of EBNA-1 with ganetespib compared to LCLa. This suggests that reduction of EBNA-1 may be more important than LMP1 for the ability of ganetespib to kill EBV-transformed B cells.
While ganetespib reduced the percentage of EBV infected cells in the peripheral blood of our patient, it did not reduce the level of EBV DNA. If ganetespib kills EBV-infected cells, viral DNA can be released from tissues or peripheral blood cells into the plasma and assays of whole blood (which includes PBMCs and plasma) may not show a change until the viral DNA is cleared. Thus, the percentage of EBV-infected B cells likely indicates the initial effect of a drug that kills virus-infected cells, while a longer course of therapy may be needed to see a change in the viral load in whole blood. In fact, patients with XMEN disease and mutations in MAGT1 who were treated with magnesium supplementation and whose level of EBV-infected cells in the blood rapidly declined [30], did not show a fall in the level of EBV DNA in whole blood until several months of continuous therapy (Chaigne-Delalande and Lenardo, unpublished data).
In our patient, it is possible that the dosing frequency of ganetespib used (once a week for two of every three weeks) was insufficient for the drug to be effective, and in some clinical studies the drug is given twice weekly. It is also likely that the drug would be more effective when used in combination with other drugs. Resistance to therapy may be less likely to occur with ganetespib and other HSP90 inhibitors than with other chemotherapeutic drugs for EBV lymphoma, since HSP90 inhibitors act on latent and lytic viral as well as cellular proteins so mutations would presumably need to occur in multiple proteins. Thus, if able to be tolerated for sufficient periods of time, ganetespib and other HSP90 inhibitors may be useful agents as part of a chemotherapeutic regimen for EBV-associated malignancies.
Acknowledgements
This work was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases and the National Cancer Institute. We thank Robert Bradley (Synta Pharmaceuticals) for planning and oversight of our clinical study, Jeffery Sample (Pennsylvania State University College of Medicine) for Mutu I, Kem I, and Akata cell lines, Paul Ling (Baylor College of Medicine) and Norio Shimizu (Tokyo Medical and Dental University) for SNT16 cells, and Harlan Pietz (Laboratory of Infectious Diseases, NIH) for statistical analyses.
References
- [1].Longnecker L, Kieff E, Cohen JI. Epstein-Barr Virus In: Knipe DM, Howley PM, Cohen JI, Griffith DE, Lamb RA, Martin MA, Racaniello V, Roizman B, editors. Fields Virology, 6th edition Philadelphia, PA: Lippincott Williams & Wilkins; 2013. p 1898–1959 [Google Scholar]
- [2].Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 2010;10:537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nagy PD, Wang RY, Pogany J, Hafren A, Makinen K. Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology. 2011. March 15;411(2):374–82. [DOI] [PubMed] [Google Scholar]
- [4].Knox C, Luke GA, Blatch GL, Pesce ER. Heat shock protein 40 (Hsp40) plays a key role in the virus life cycle. Virus Res 2011;160:15–24. [DOI] [PubMed] [Google Scholar]
- [5].Cheung RK, Dosch HM. The growth transformation of human B cells involves superinduction of hsp70 and hsp90. Virology 1993;193:700–8. [DOI] [PubMed] [Google Scholar]
- [6].Kotsiopriftis M, Tanner JE, Alfieri C. Heat shock protein 90 expression in Epstein-Barr virus-infected B cells promotes gammadelta T-cell proliferation in vitro. J Virol 2005;79:7255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alsayed Y, Leleu X, Leontovich A, et al. Proteomics analysis in post-transplant lymphoproliferative disorders. Eur J Haematol 2008;81:298–303. [DOI] [PubMed] [Google Scholar]
- [8].Sun X, Barlow EA, Ma S, et al. Hsp90 inhibitors block outgrowth of EBV-infected malignant cells in vitro and in vivo through an EBNA1-dependent mechanism. Proc Natl Acad Sci U S A 2010;107:3146–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Murata T, Iwata S, Siddiquey MN, et al. Heat shock protein 90 inhibitors repress latent membrane protein 1 (LMP1) expression and proliferation of Epstein-Barr virus-positive natural killer cell lymphoma. PLoS One 2013;8:e63566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suzuki M, Takeda T, Nakagawa H, et al. The heat shock protein 90 inhibitor BIIB021 suppresses the growth of T and natural killer cell lymphomas. Front Microbiol 2015;6:280- [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jeon YK, Park CH, Kim KY, et al. The heat-shock protein 90 inhibitor, geldanamycin, induces apoptotic cell death in Epstein-Barr virus-positive NK/T-cell lymphoma by Akt down-regulation. J Pathol 2007;213:170–9. [DOI] [PubMed] [Google Scholar]
- [12].Chan KC, Ting CM, Chan PS, et al. A novel Hsp90 inhibitor AT13387 induces senescence in EBV-positive nasopharyngeal carcinoma cells and suppresses tumor formation. Mol Cancer 2013;12:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sun X, Bristol JA, Iwahori S, et al. Hsp90 inhibitor 17-DMAG decreases expression of conserved herpesvirus protein kinases and reduces virus production in Epstein-Barr virus-infected cells. J Virol 2013;87:10126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nayar U, Lu P, Goldstein RL, et al. Targeting the Hsp90-associated viral oncoproteome in gammaherpesvirus-associated malignancies. Blood 2013;122:2837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takada K, Horinouchi K, Ono Y, et al. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes 1991;5,147–156. [DOI] [PubMed] [Google Scholar]
- [16].Gregory CD, Rowe M, Rickinson AB. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt’s lymphoma cell line. J Gen Virol 1990;71:1481–95. [DOI] [PubMed] [Google Scholar]
- [17].Menezes J, Leibold W, Klein G, Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt’s lymphoma. Biomedicine 1975;22:276–84. [PubMed] [Google Scholar]
- [18].Calender A, Billaud M, Aubry JP, Banchereau J, Vuillaume M, Lenoir GM. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A 1987;84:8060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang Y, Nagata H, Ikeuchi T, et al. Common cytological and cytogenetic features of Epstein-Barr virus (EBV)-positive natural killer (NK) cells and cell lines derived from patients with nasal T/NK-cell lymphomas, chronic active EBV infection and hydroa vacciniforme-like eruptions. Br J Haematol 2003;121:805–814. [DOI] [PubMed] [Google Scholar]
- [20].Ramakrishnan R, Donahue H, Garcia D, et al. Epstein-Barr virus BART9 miRNA modulates LMP1 levels and affects growth rate of nasal NK T cell lymphomas. PLoS One 2011;6(11):e27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Klion AD, Mejia R, Cowen EW, et al. Chronic active Epstein-Barr virus infection: a novel cause of lymphocytic variant hypereosinophilic syndrome. Blood 2013;121:2364–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Calattini S, Sereti I, Scheinberg P, Kimura H, Childs RW, Cohen JI. Detection of EBV genomes in plasmablasts/plasma cells and non-B cells in the blood of most patients with EBV lymphoproliferative disorders by using Immuno-FISH. Blood 2010;116:4546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rowe M, Young LS, Crocker J, et al. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med 1991;173:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 1998;94:471–480. [DOI] [PubMed] [Google Scholar]
- [25].Jhaveri K, Modi S. Ganetespib: research and clinical development. Onco Targets Ther 2015;8:1849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Proia DA, Bates RC. Ganetespib and HSP90: translating preclinical hypotheses into clinical promise. Cancer Res 2014;74:1294–300. [DOI] [PubMed] [Google Scholar]
- [27].Nagaraju GP, Mezina A, Shaib WL, Landry J, El-Rayes BF. Targeting the Janus-activated kinase-2-STAT3 signalling pathway in pancreatic cancer using the HSP90 inhibitor ganetespib. Eur J Cancer. 2016;52:109–19. [DOI] [PubMed] [Google Scholar]
- [28].Friedland JC, Smith DL, Sang J, Acquaviva J, He S, Zhang C, Proia DA. Targeted inhibition of Hsp90 by ganetespib is effective across a broad spectrum of breast cancer subtypes. Invest New Drugs. 2014;32:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu X, Marmarelis ME, Hodi FS. Activity of the heat shock protein 90 inhibitor ganetespib in melanoma. PLoS One. 2013;8:e56134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chaigne-Delalande B, Li FY, O’Connor GM, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 2013;341:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


