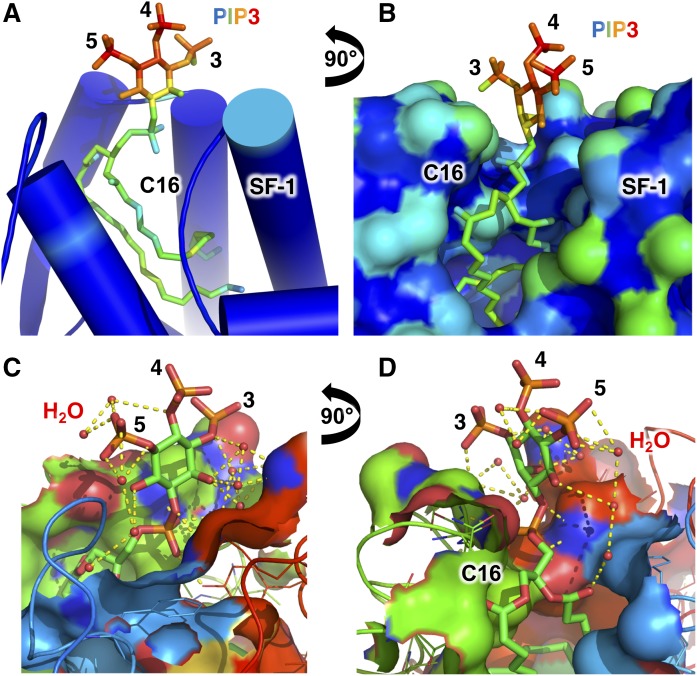
Fig. 2.
Solvent accessibility of the SF-1 bound PI(3,4,5)P3 headgroup is high and participates in an extensive water-mediated hydrogen bonding network. A: Cartoon representation of the crystal structure of SF-1 bound to dipalmitoyl PI(3,4,5)P3 (13) colored as a spectrum to solvent accessibility, where the least solvent accessible is blue<green<yellow<orange<red is most solvent accessible. Note the very high solvent accessibility of the PI(3,4,5)P3 headgoup phosphorylations on the indicated 3, 4, and 5 positions, and the low solvent accessibility of the di-C16 palmitoyl acyl chains. B: Surface representation and orthogonal view of A with identical coloring scheme, again showing high solvent accessibility of the PI(3,4,5)P3 phosphorylations even relative to hydrophilic surface residues on the SF-1 protein. C, D: Orthogonal ligand-sites surface cutaway views of the crystal structure of SF-1 bound to dipalmitoyl PI(3,4,5)P3, showing the extensive water-mediated hydrogen bonding network the 3, 4, and 5 phosphorylations participate in. Water molecules are shown as small red spheres. The crystal structure of SF-1 bound to PI(4,5)P2 is very similar in terms of the solvent accessibility of the phosphoinositide headgroup [Protein Data Bank (PDB): 4QK4]. The high solvent accessibility of PIP3 bound to SF-1 highlights that the headgroup in nuclear phosphoinositide signaling complexes can be readily accessible for catalysis and other signaling events.