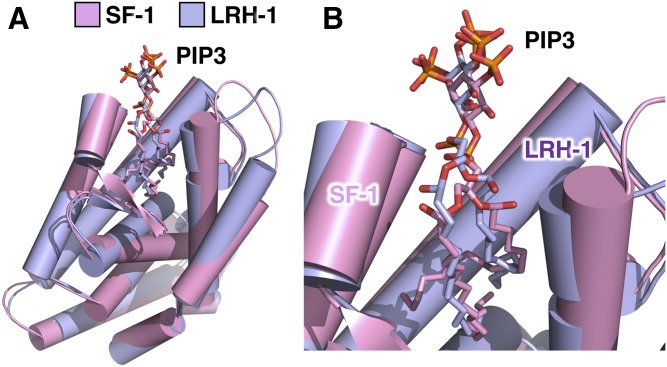
Fig. 6.
Human LRH-1 and SF-1 both bind nuclear PIP3 in very similar ways. A: Superposition of the 1.8 Å crystal structure of human LRH-1 ligand binding domain (14) (represented as purple cartoon) and the crystal structure of human SF-1 ligand binding domain (13) (represented as pink cartoon), each bound individually to one dipalmitoyl PI(3,4,5)P3 molecule. The two superposed structures have a root mean square deviation of less than 1 Å (0.898 Å) that, while totally identical, shows the high degree of homology between SF-1 and LRH-1. B: Close-up of A showing similar solvent accessibility of PI(3,4,5)P3 headgroups when the phosphoinositide is bound to either human SF-1 or human LRH-1. This structural comparison suggests that the same structural format of PI(3,4,5)P3 that grants special signaling capacity to SF-1 is conserved within LRH-1. It remains to be determined whether IPMK or PTEN can directly remodel phosphoinositides bound to LRH-1.