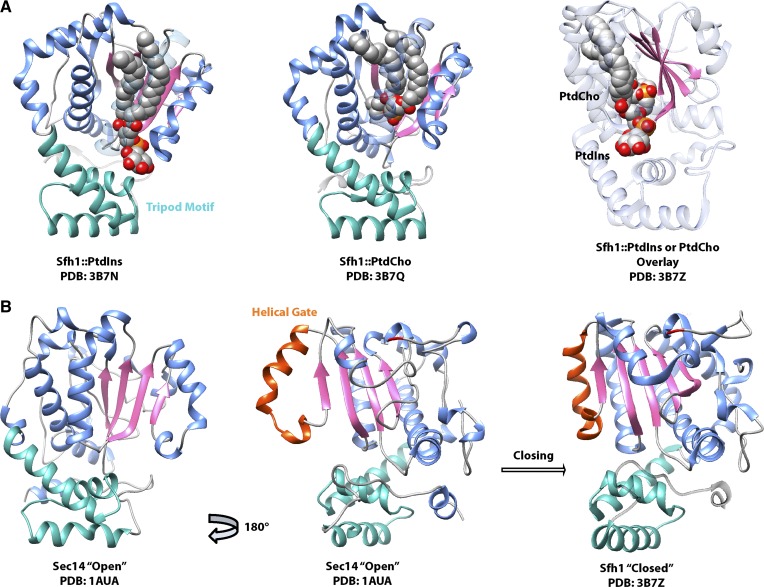
Fig. 3.
The Sec14 structure. Crystal structures of Sec14 and the Sec14-like PITP Sfh1 are shown, with the tripod motif (green) oriented as if toward the membrane. The lipid binding β-sheet floor is rendered in pink, and the helices that line the binding pocket are in blue. A: Crystal structures of the Sec14-like PITP Sfh1 reveal that PtdIns and PtdCho both bind in the lipid binding pocket, with the PtdCho headgroup buried deep within the pocket and the PtdIns headgroup facing outwards toward the protein surface. Overlay of Sfh1 bound to either lipid illustrates that PtdIns and PtdCho acyl chain binding regions overlap, and Sfh1 cannot fully accommodate both lipids at the same time. B: The crystal structure of open lipid-free Sec14 is compared with Sfh1 bound to PtdCho (lipid not shown for clarity). The helical gate (orange) closes around the lipid binding pocket, with a key residue of the G-module (G266) highlighted in red.